Kasugamycin residue protein united Chinese herbal medicine preparation for resisting WSSV (white spot syndrome virus) and application of kasugamycin residue protein united Chinese herbal medicine preparation
A technology of kasugamycin and Chinese herbal medicine, which is applied in the field of kasugamycin residue protein combined with Chinese herbal medicine preparations, can solve the problem of insignificant drug efficacy, achieve the effects of controlling WSSV damage to prawns, improving product quality, survival rate and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] After the aforementioned steps of protein and polypeptide separation and purification, the protein of the residue is separated from other substances of the residue such as metal ions, lipids, sugars, and cellulose to obtain the kasugamycin residue protein. After testing, the Kasugamycin In mycin residue protein, by mass ratio:
[0059] 28.4kDa: 40-50%
[0060] 31.0kDa: 20-30%
[0061] 14.4kDa: 10-20%,
[0062] 58.9kDa: 10-20%.
[0063] The above-mentioned kasugamycin residue protein is regarded as the kasugamycin residue protein group.
[0064] The components of the Chinese herbal medicine group are: 10 parts of honeysuckle, 15 parts of forsythia, 25 parts of Radix Radix, 20 parts of Astragalus, 15 parts of Folium Folium, 10 parts of Cuscuta, and 5 parts of crude protein.
[0065] The composition of the kasugamycin residue protein and Chinese herbal medicine interworking group is: the above-mentioned kasugamycin residue protein and the above-mentioned Chinese herbal...
Embodiment 2
[0068] To determine the protein content of the kasugamycin residue, the following steps were used:
[0069] ①Preparation of standard protein solution: Accurately weigh 500mg of bovine serum albumin, dilute to 50mL with distilled water, and make 1L of 10mg / mol solution. Take 1mL of this solution and dilute it to 10mL with deionized water to prepare a 1.00mg / mL standard protein solution. ② Preparation of Coomassie Brilliant Blue G-250 solution: Accurately weigh 100 mg of Coomassie Brilliant Blue G-250, dissolve in 50 mL of 95% ethanol, add 120 mL of 85% phosphoric acid, dilute to 1000 mL for later use. ③Drawing of the standard curve: Dilute the standard protein (initial concentration: 1.00mg / mL) successively to 0, 0.2, 0.4, 0.6, 0.8 and 1.00mg / mL protein concentration. After mixing evenly, add 5mL test solution to each tube. Shake the Masie Brilliant Blue solution, and let it stand for about 5 minutes. Use a cuvette with a light path of 1 cm to measure the absorbance at 595 nm....
Embodiment 3
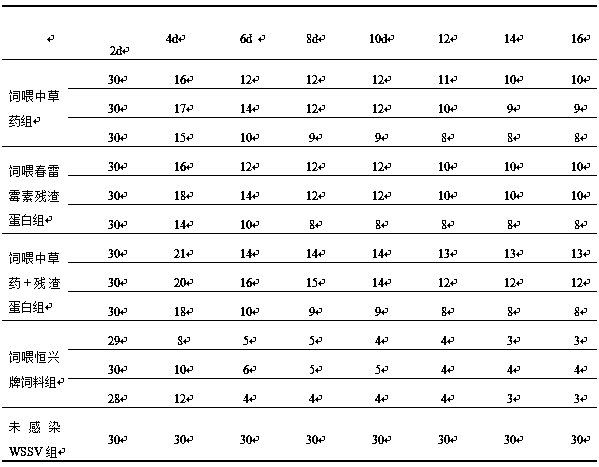
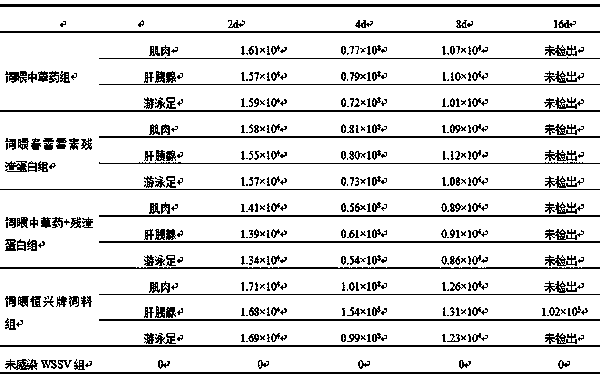
[0071] In order to compare the mortality of each group after WSSV injection in different feeding groups, several tails of Litopenaeus vannamei with typical symptoms of WSSV were selected from the WSSV shrimp pond, and WSSV was positive by PCR detection.
[0072] For diseased shrimp, remove the head, thorax and hepatopancreas, and cut them into pieces under ice bath conditions. Add 100m L of PBS buffer solution per gram of tissue, the pH of the buffer solution is about 7.6, homogenize in an ice bath for 1 hour, filter the tissue fluid through a 200-mesh silk screen through the homogenate, and then filter through a 0.45 μm microporous membrane, Obtain filtrate, and this filtrate just is free WSSV crude extraction mother liquor, then gets this mother liquor 1mL, dilutes this mother liquor with PBS damping fluid 10, 10 -4The diluent was intramuscular injection of prawns, and the experimental group was fed with Chinese herbal medicine for 7 days, fed with kasugamycin residue for 7 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com