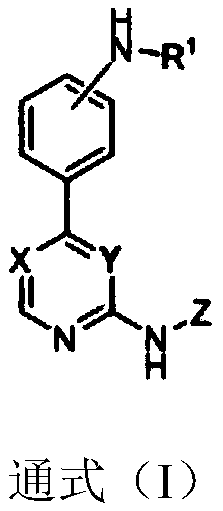
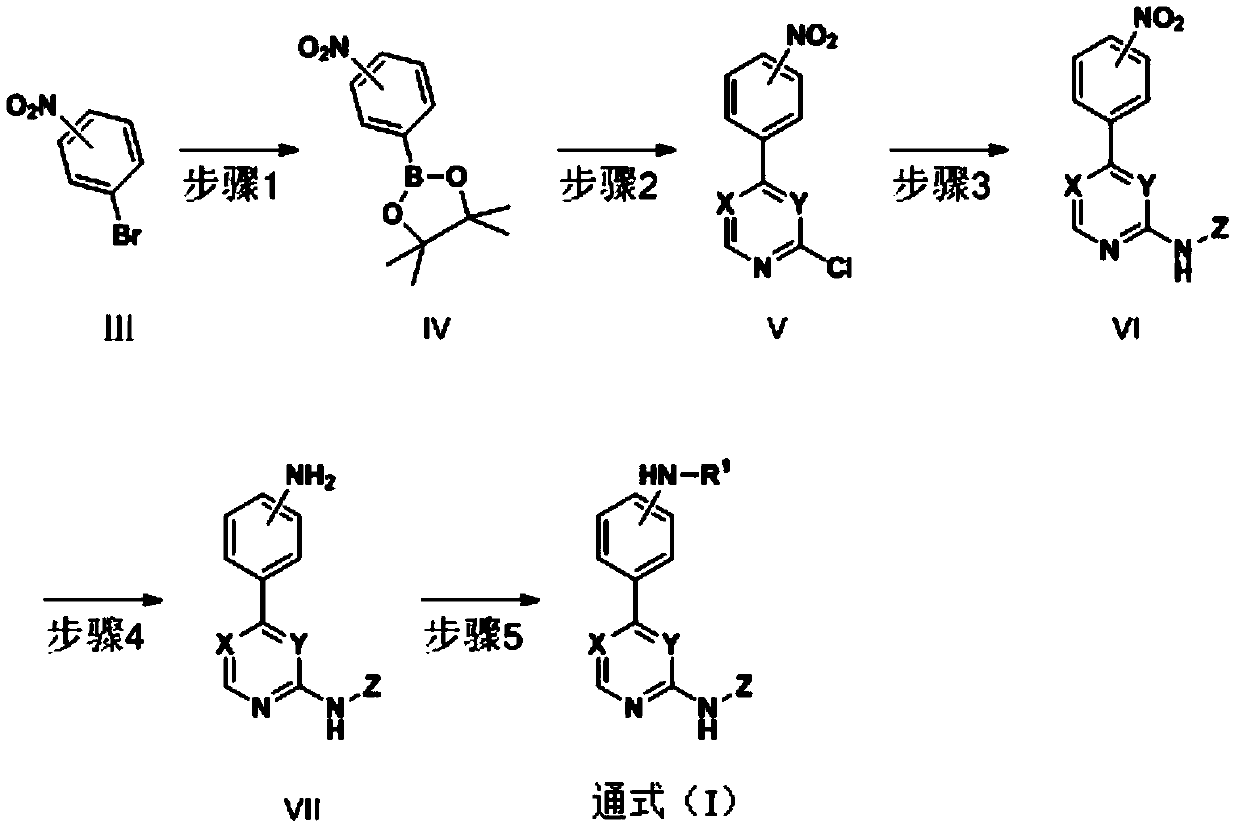
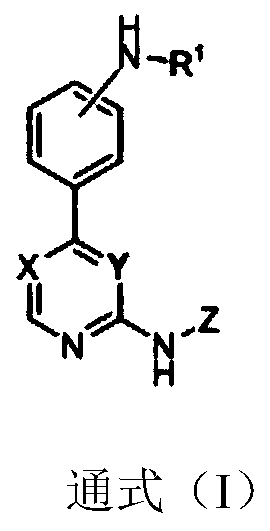
Substitutive phenyl pyrimidine derivative as JAK kinase inhibitor or medicinal salt, preparation method and application thereof
A phenylpyrimidine-like and kinase inhibitor technology, applied in the field of kinase inhibitors, can solve problems such as the inability to regulate gene expression in the nucleus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] N-(3-(6-((1-(2-methoxyethyl)-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)phenyl)acrylamide
[0113]
[0114] A) 4-Chloro-6-(3-nitrophenyl)pyrimidine
[0115] To 3-bromonitrobenzene (1 g) and 50 mL of anhydrous 1,4-dioxane at room temperature, were added bis-pinacol borate (1.34 g) and KOAc (1.48 g) over catalyst Pd ( PPh 3 ) 4 (3%) and react at 100° C. for 12 h under anaerobic conditions. Cool to room temperature, add 4,6-dichloropyrimidine (1.07g) to the mixed solution, 2M K 2 CO 3 Aqueous solution and Pd(PPh 3 ) 4 (3%) The temperature was raised to 100° C. to ensure anaerobic conditions to continue the reaction for 12 hours. Cool to room temperature, filter with celite, dry under reduced pressure, and purify by silica gel column chromatography to obtain the title compound (0.93 g).
[0116] 1 H NMR (300MHz, CDCl 3 ( t, J=8.0Hz, 1H).
[0117] B) 6-(3-nitrobenzene)-N 4 -(1-(2-Methoxyethyl)-1H-pyrazol-4-yl)-4-aminopyrimidine
[0118] Add 1-(2-methoxyethyl)-1H-p...
Embodiment 2 to 23
[0128] In Examples 2 to 23, the same method as in Example 1 was used.
[0129] Wherein embodiment 3, 5, 7, 9, 15, 16, 19 and 20 in step A) 3-bromonitrobenzene (1g) is correspondingly replaced with 4-bromonitrobenzene (1g) and other conditions remain unchanged.
[0130] According to the structural formula of the embodiment, the step B) corresponds to different aminoalkylated substituents, specifically the Z substituent (described above).
[0131] Among them, in Examples 13-23, corresponding to step D), dropwise addition of 1 mL of acryloyl chloride diluted in dry DCM at 0°C was replaced by dropwise addition of 1 mL of crotonyl chloride diluted in dry DCM at 0°C, and other conditions remained unchanged.
[0132] The above molar equivalent ratio is the same as the corresponding reaction equivalent of Example 1.
[0133] The following title compounds can then be obtained, see Table 2. MS in this table means the measured value.
[0134] Table 2 Structure and NMR data of some com...
Embodiment 24
[0143] 2-cyano-N-(3-(6-((1-methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)phenyl)acetamide
[0144]
[0145] Experimental steps A), B), and C) utilize the same method as in Example 1 to obtain 6-(3-aminobenzene)-N 4 -(1-Methyl-1H-pyrazol-4-yl)-4-aminopyrimidine.
[0146] E) 2-cyano-N-(3-(6-((1-methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)phenyl)acetamide
[0147] 6-(3-Aminobenzene)-N 4 -(1-Methyl-1H-pyrazol-4-yl)-4-aminopyrimidine (0.12g,), cyanoacetic acid (0.04g), DIEA (0.13g) and 15mL dry THF, HATU was added under stirring at 0°C (0.37g) was stirred and reacted for 30min, then turned to room temperature and stirred for reaction. After the reaction was completed, 20 mL of ethyl acetate was added, and 10% citric acid aqueous solution was added to saturate NaHCO 3 The aqueous solution was washed twice, the organic phase was mixed, and the title compound was obtained by separation and purification by silica gel column chromatography.
[0148] 1 H NMR (300MHz, DMSO) δ8.66(s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com