Application of 4-methyl-5,6-dihydropyran-2ketone in preparing anti-hepatic fibrosis and anti-hepatoma drugs
An anti-hepatic fibrosis and dihydropyran technology, applied in the direction of anti-tumor drugs, drug combinations, digestive system, etc., can solve problems such as poor prognosis and limited treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention has no special requirements on the source of the 4-methyl-5,6-dihydropyran-2-one, use commercially available 4-methyl-5,6-dihydropyran-2-one or Can be prepared. When it is necessary to prepare 4-methyl-5,6-dihydropyran-2-one by itself, the preparation method of 4-methyl-5,6-dihydropyran-2-one preferably includes the following steps:
[0028] Under the action of a catalyst, the mevalonolactone is eliminated to obtain 4-methyl-5,6-dihydropyran-2-one.
[0029] In the present invention, the catalyst is preferably p-toluenesulfonic acid monohydrate; the mass ratio of the mevalonolactone to the catalyst is preferably 20:1; in the present invention, the solvent used in the elimination reaction is preferably Toluene, the present invention has no special requirements on the amount of the solvent, as long as the mevalonolactone and the catalyst can be dissolved. In the present invention, the temperature of the elimination reaction is preferably the reflux ...
Embodiment 1
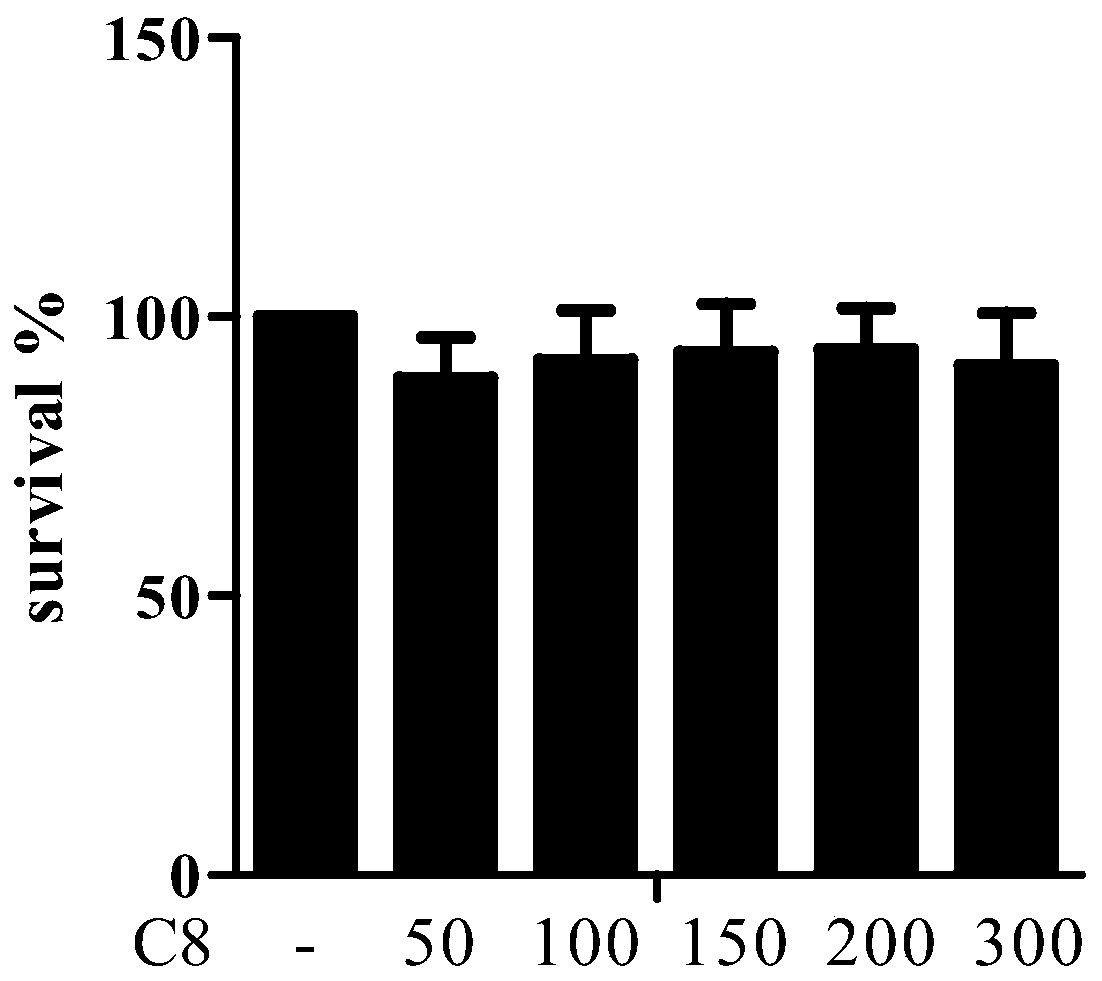
[0042] Example 1 4-Methyl-5,6-dihydropyran-2one has no toxicity to LX2 cells
[0043] Using different concentrations of 4-methyl-5,6-dihydropyran-2-one (hereinafter referred to as C8) (50, 100, 150, 200, 300 μmol L -1 ) Treat human hepatic stellate cells LX2 in the logarithmic growth phase for 24 hours, and use the sulforhodamine B protein staining (SRB) colorimetric method to detect the effect of the compound on cell proliferation. The specific operation method is as follows:
[0044] (1) Cell plating: cells in the logarithmic growth phase were seeded in 96-well culture plates, at 37°C, 5% CO 2 Cultivate under the condition for 24h;
[0045] (2) Administration: After the cells adhere to the wall, the original medium is discarded, and the compound stock solution is prepared into a gradient concentration with the medium, and the blank control group, the vehicle control group, and the administration group are respectively set up, and each concentration is set to 3 Duplicate we...
Embodiment 2
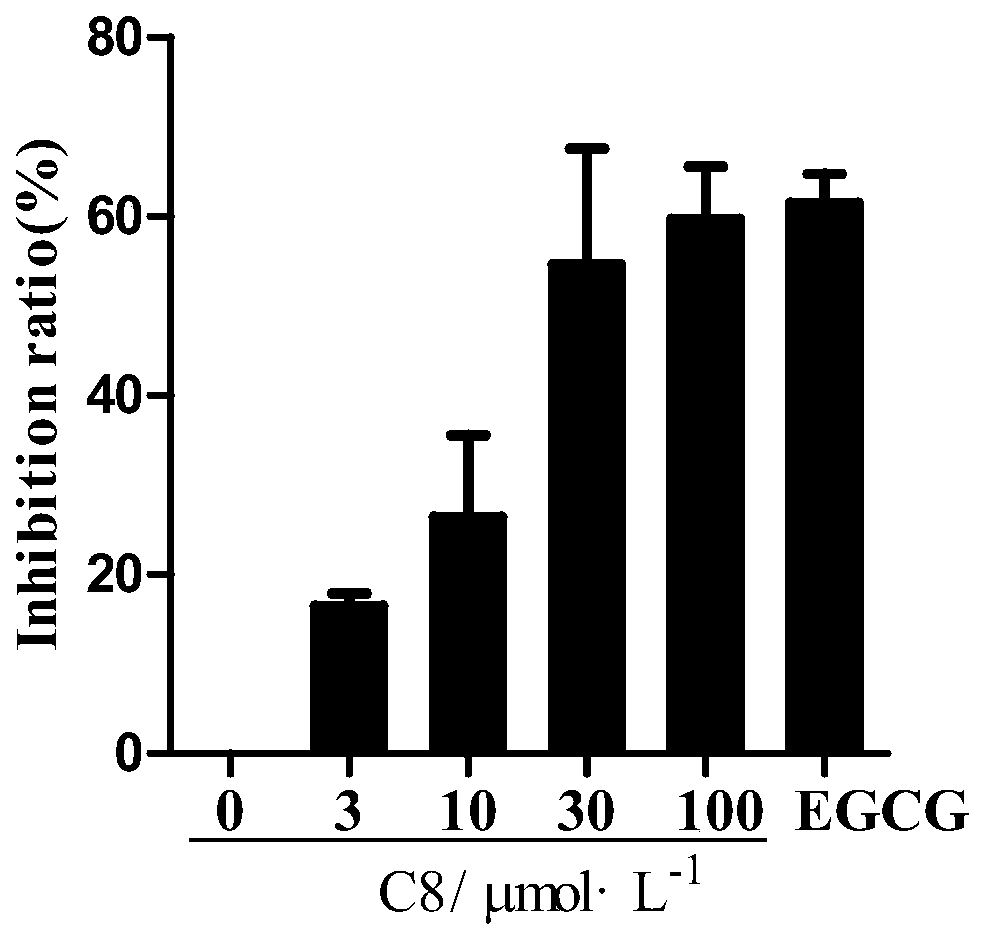
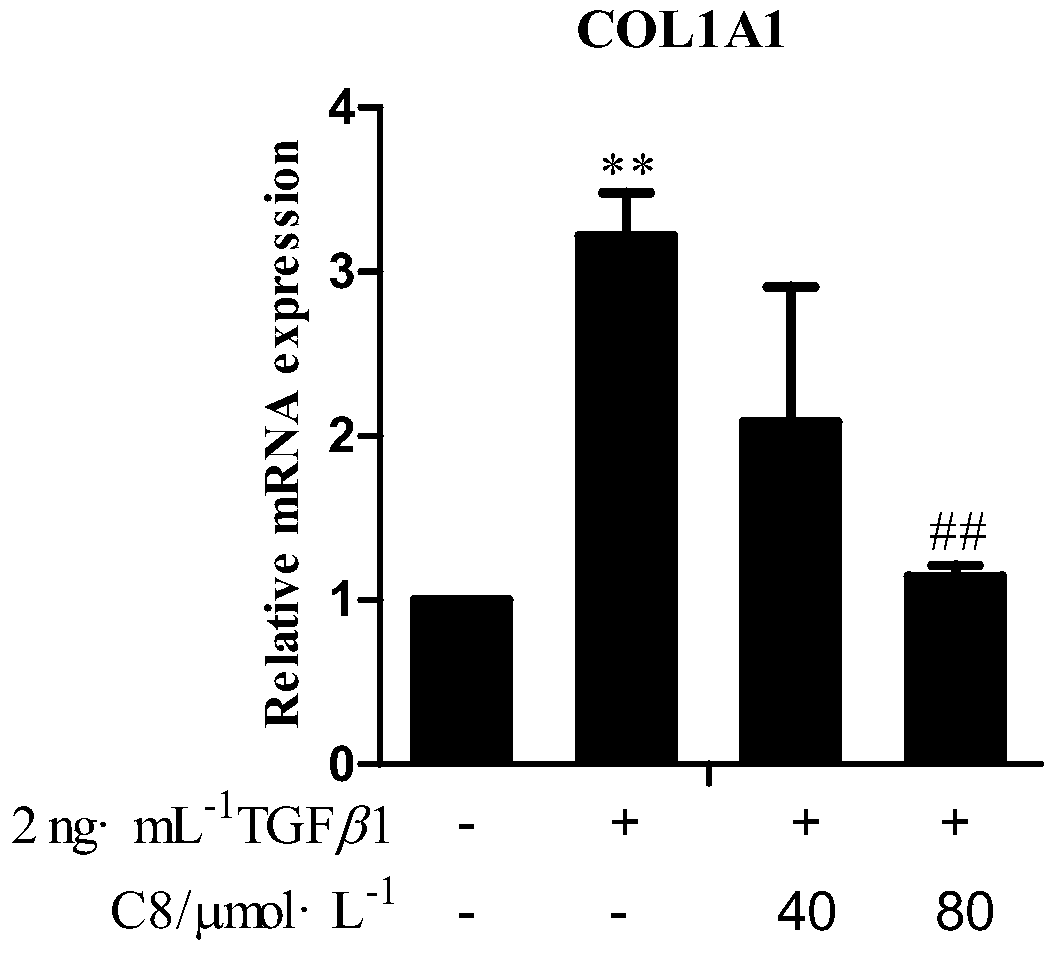
[0052] Example 2 4-methyl-5,6-dihydropyran-2one inhibits the activity of COL1A1 promoter in hepatic stellate cells
[0053] The most important feature of liver fibrosis is the overexpression of collagen, especially type I collagen α1 (COL1A1). High expression is manifested at the transcriptional level. If the promoter activity can be inhibited, collagen production can be inhibited, thereby inhibiting liver fibrosis. Therefore, the effect of 4-methyl-5,6-dihydropyran-2one on the activity of collagen promoter was used as an index to initially judge the anti-hepatic fibrosis effect of potential drugs.
[0054] Using a high-throughput anti-hepatic fibrosis screening model based on the COL1A1 promoter, a single luciferase gene reporter assay system was used to detect different concentrations of 4-methyl-5,6-dihydropyran-2-one (3, 20, 50 and 100μmol·L -1 ) and positive control 25 μmol L -1 Indicate the impact of gallocatechin gallate (EGCG) on the activity of the COL1A1 promoter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com