Method for reducing ratio of magnesium to lithium in salt lake through solar-assisted salt crystallization
A magnesium-lithium ratio and salt lake technology, applied in the direction of lithium carbonate;/acid carbonate, magnesium chloride, magnesium halide, etc., can solve the problems of long production cycle, high power and water consumption of adsorption method, and high corrosion. problems, to achieve the effect of saving manpower and material resources, shortening the production cycle, and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
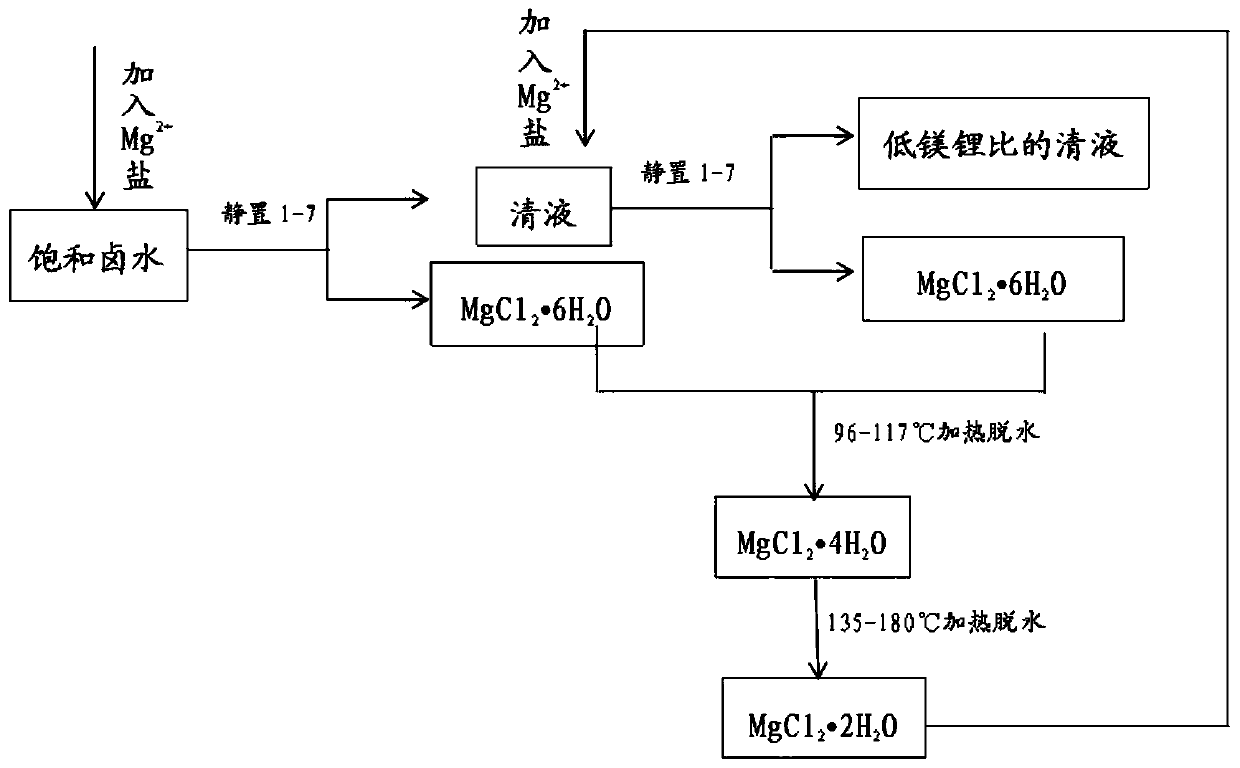
[0028] (1) At room temperature, in the primary salt field of magnesium-lithium saturated brine with a magnesium-lithium ratio of 80:1, according to Mg 2+ Mg in salt and saturated brine 2+ Add Mg to the molar ratio of 1:8 2+ Salt, dry the salt in the salt field for 4 days to get clear liquid and granular precipitated crystal MgCl 2·6H 2 O.
[0029] (2) Introduce the clear liquid into the secondary salt field, collect the precipitated crystal MgCl 2 ·6H 2 O, MgCl 2 ·6H 2 O is dehydrated at 105°C to obtain MgCl 2 4H 2 O, MgCl 2 4H 2 O is heated to 150°C for dehydration to obtain MgCl 2 2H 2 O.
[0030] (3) According to Mg 2+ Mg in the supernatant solution of salt and step (2) 2+ The molar ratio is 1:8 plus Mg 2+ Salt in secondary salt pans, where Mg 2+ Salt contains all of the MgCl prepared in step (2) 2 2H 2 O, the clear liquid was sun-salted for 4 days, and the clear liquid and granular precipitated crystal MgCl were obtained again 2 ·6H 2 O.
[0031] (4) ...
Embodiment 2
[0034] (1) At room temperature, in the primary salt field of magnesium-lithium saturated brine with a magnesium-lithium ratio of 200:1, according to Mg 2+ Mg in salt and saturated brine 2+ Add Mg to the molar ratio of 1:5 2+ Salt, dry the salt in the salt field for 7 days to obtain clear liquid and granular precipitated crystal MgCl 2 ·6H 2 O.
[0035] (2) Introduce the clear liquid into the secondary salt field, collect the precipitated crystal MgCl 2 ·6H 2 O, MgCl 2 ·6H 2 O is heated to 117°C for dehydration to obtain MgCl 2 4H 2 O, MgCl 2 4H 2 O is heated to 200°C for dehydration to obtain MgCl 2 2H 2 O.
[0036] (3) According to Mg 2+ Mg in the supernatant solution of salt and step (2) 2+ The molar ratio is 1:5 plus Mg 2+ Salt in secondary salt pans, Mg 2+ Salt contains all of the MgCl prepared in step (2) 2 2H 2 O, the clear liquid was sun-salted for 7 days, and the clear liquid and granular precipitated crystal MgCl were obtained again 2 ·6H 2 O.
...
Embodiment 3
[0039] (1) At room temperature, in the primary salt field of magnesium-lithium saturated brine with a magnesium-lithium ratio of 10:1, according to Mg 2+ Mg in salt and saturated brine 2+ Add Mg to the molar ratio of 1:10 2+ Salt, dry the salt in the salt field for 1 day to get clear liquid and granular precipitated crystal MgCl 2 ·6H 2 O.
[0040] (2) Introduce the clear liquid into the secondary salt field, collect the precipitated crystal MgCl 2 ·6H 2 O, MgCl 2 ·6H 2 O is heated to 96°C and dehydrated to obtain MgCl 2 4H 2 O, MgCl 2 4H 2 O is heated to 135°C for dehydration to obtain MgCl 2 2H 2 O.
[0041] (3) According to Mg 2+ Mg in the supernatant solution of salt and step (2) 2+ The molar ratio is 1:10 plus Mg 2+ Salt in secondary salt pans, where Mg 2+ Salt contains all of the MgCl prepared in step (2) 2 2H 2 O, the clear liquid is salted for 1 day, and the clear liquid and granular precipitated crystal MgCl are obtained again 2 ·6H 2 O.
[0042]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com