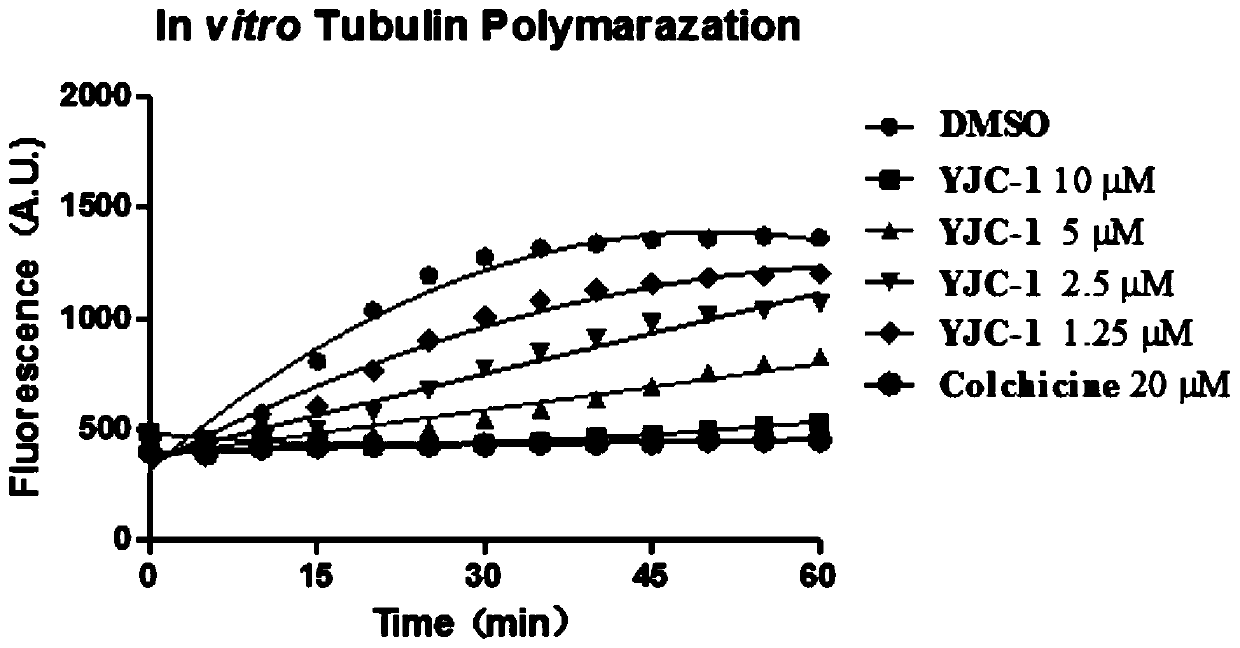
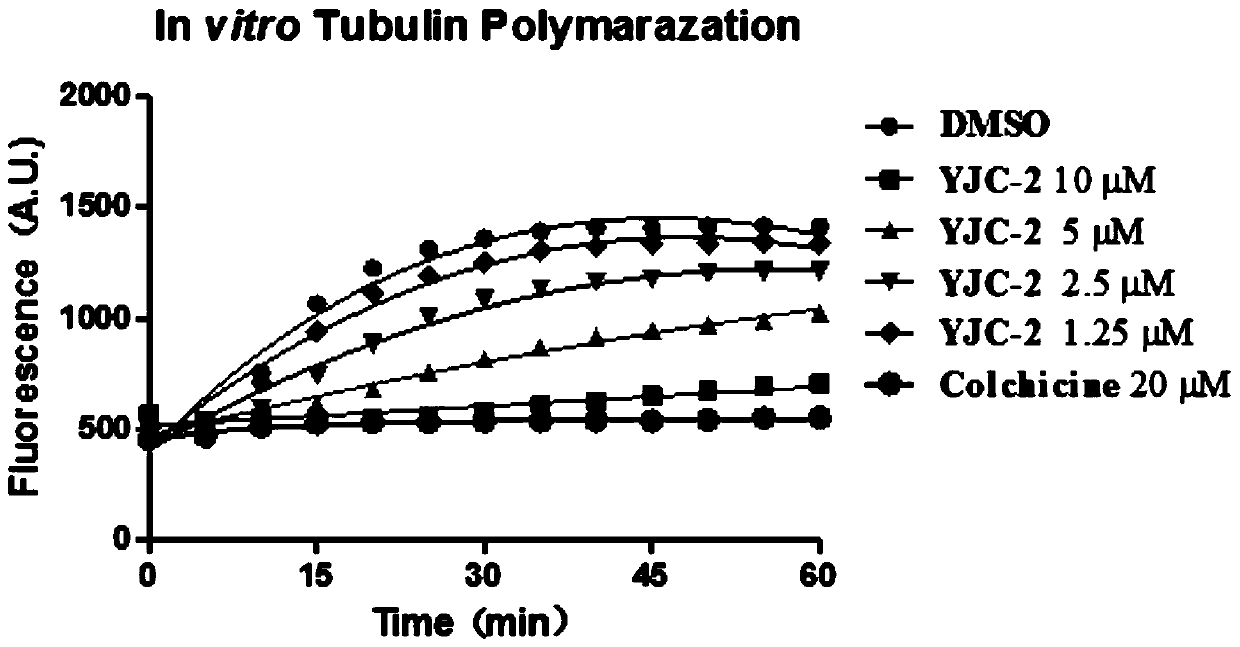
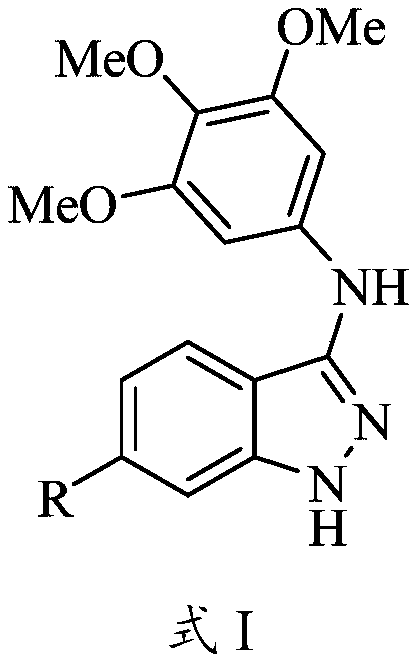
Tubulin inhibitor and preparation method and application thereof
A pharmaceutical preparation and reaction technology, applied in the field of medicinal chemistry, can solve the problems of easy drug resistance, high toxicity and side effects, and difficult synthesis, and achieve the effects of novel structure, reduction of toxicity and side effects, and enhanced specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] In yet another specific embodiment of the present invention, the preparation method includes the following steps:
[0038] With 2-fluorobenzoic acid containing different substituents at the 4-position as the starting material, react with 3,4,5-trimethoxyaniline to obtain 2-fluoro-N-(3,4, 5-trimethoxyphenyl) benzamide (formula 2), the compound of formula 2 undergoes a thiolation reaction under the effect of Lawson's reagent to obtain 2-fluoro-N-(3,4,5- Trimethoxyphenyl) thiobenzamide (formula 3), the compound of formula 3 is cyclized with hydrazine hydrate to generate the compound shown in formula I.
[0039] In yet another specific embodiment of the present invention, when R is taken from a methyl group, the preparation method includes the following steps:
[0040] (1) Dissolve 2-fluoro-4-methylbenzoic acid in N,N-dimethylformamide, add triethylamine, 3,4,5-trimethoxyaniline, 2-(7-benzene oxide Triazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate, stirred, added...
Embodiment 1
[0068] Embodiment 1: the preparation of structural formula 1 compound
[0069] (1) Dissolve 2-fluoro-4-methylbenzoic acid (50mg) in N,N-dimethylformamide (4mL), add triethylamine (70μL), 3,4,5-trimethoxy Aniline (66mg), 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (137mg), reacted for 2h, added water, extracted with ethyl acetate, Column chromatography gave 2-fluoro-4-methyl-N-(3,4,5-trimethoxyphenyl)benzamide (100 mg) with a yield of 96%. Melting point: 117-118°C.
[0070] (2) Dissolve 2-fluoro-4-methyl-N-(3,4,5-trimethoxyphenyl)benzamide (80mg) in toluene (4mL), add Lawson's reagent (71mg), 105 °C, spin to dry the solvent, and perform column chromatography to obtain crude 2-fluoro-4-methoxy-N-(3,4,5-trimethoxyphenyl)thiobenzamide.
[0071] (3) Dissolve the crude 2-fluoro-4-methyl-N-(3,4,5-trimethoxyphenyl)thiobenzamide obtained in step (2) in dimethyl sulfoxide, add hydration Hydrazine (167 μL), react at 110°C, add water, and filter with suc...
Embodiment 2
[0073] Embodiment 2: the preparation of structural formula 2 compound
[0074] (1) Dissolve 2-fluoro-4-methoxybenzoic acid (80mg) in N,N-dimethylformamide (4mL), add triethylamine (100μL), 3,4,5-trimethoxy Aniline (95mg), 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (196mg), reacted for 2h, added water, extracted with ethyl acetate , and column chromatography, 2-fluoro-4-methoxy-N-(3,4,5-trimethoxyphenyl)benzamide (157 mg) was obtained with a yield of 99.6%. Melting point: 126-127°C.
[0075] (2) Dissolve 2-fluoro-4-methoxy-N-(3,4,5-trimethoxyphenyl)benzamide (80mg) in toluene (4mL), add Lawson's reagent (67mg), Spin to dry the solvent at 105°C and perform column chromatography to obtain crude 2-fluoro-4-methoxy-N-(3,4,5-trimethoxyphenyl)thiobenzamide.
[0076] (3) Dissolve the crude 2-fluoro-4-methoxy-N-(3,4,5-trimethoxyphenyl)thiobenzamide obtained in step (2) in dimethyl sulfoxide, add Hydrazine hydrate (144μL), react at 110°C, add water, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com