Method for preparing pET-28a-SUMO-coagulation factor II protein antigen and polyclonal antibody thereof
A pet-28a-sumo-, polyclonal antibody technology, applied in the field of molecular biology, can solve the problem that the role and expression rules are not fully studied and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
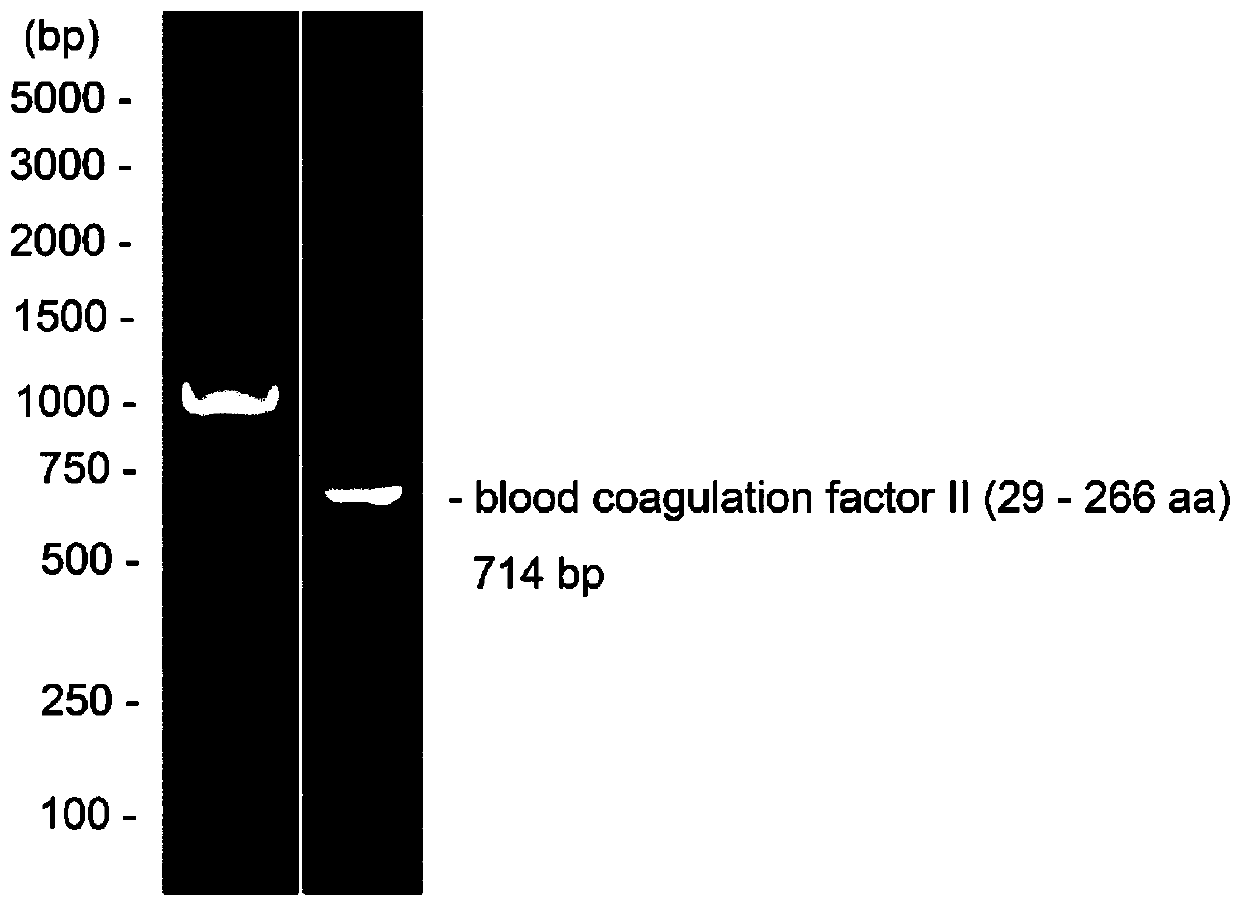
[0024] Synthesis of embodiment 1 grass carp coagulation factor II gene fragment
[0025] Based on the grass carp blood coagulation factor II gene sequence (retrieval number KF937688) in the GenBank database of the NCBI website, after using Beacon Desiner software to obtain potential primers, Oligo 6.0 was used to evaluate and finally determine the target gene sequence amplification primers, and then according to the vector homology arm sequence, enzyme The order of the cleavage site and the target gene sequence amplification primer constitutes the primer sequence (primers are synthesized by Wuhan Aibatek Biotechnology Co., Ltd.) that is finally used to amplify the grass carp coagulation factor II gene, wherein the enzyme cleavage sites are NdeI-NotI and Kan+, the primer sequence is as follows:
[0026] pET-28a-SUMO-FII-F: 5'-GAACAGATTGGTGGATCCAATAATAAGGAAGCCCTCT-3', SEQ ID NO.1;
[0027] pET-28a-SUMO-FII-R: 5'-TGCGGCCGCAAGCTTTCCTCCCACAATTCTGC-3', SEQ ID NO.2.
[0028] Using ...
Embodiment 2
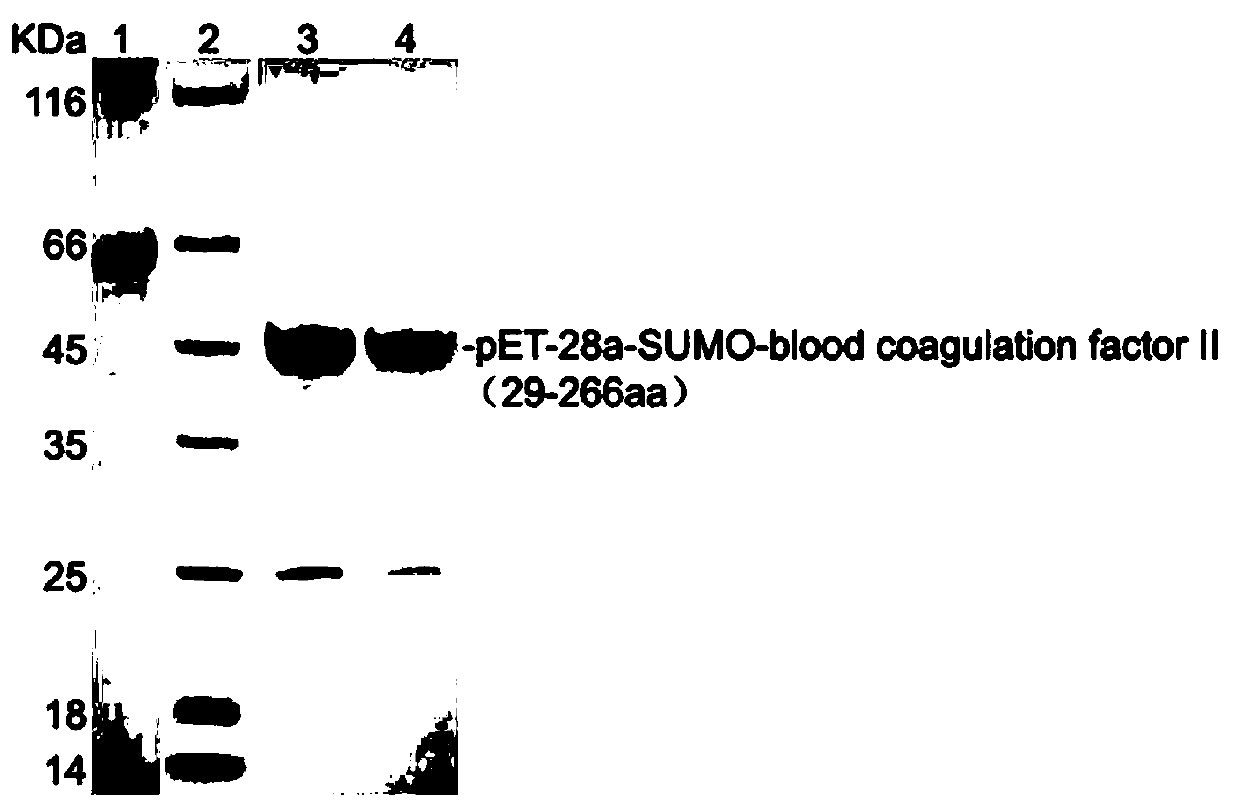
[0029] Example 2 Obtaining of recombinant protein pET-28a-SUMO-coagulation factor II antigen
[0030] The amplified grass carp coagulation factor II gene fragment that meets the expectations was purified using the AxyPrepDNA gel recovery kit from Axygen Company, and was purified according to the instructions of the pET-28a-SUMO expression vector (purchased from Huayueyang Biological (Beijing) Technology Co., Ltd.) The purified grass carp blood coagulation factor II gene fragment was connected to the pET-28a-SUMO expression vector. After sequencing to confirm that the connection was correct, it was introduced into Escherichia coli competent cell strain E.coli Rosetta and cultivated to OD 600 0.5-0.6. Then add 0.8mM IPTG, induce for 4 hours at 37°C to achieve bacteriostasis, and use SDS-PAGE gel electrophoresis to purify the target protein to obtain pET-28a-SUMO-coagulation factor II protein (pET-28a-SUMO-coagulation factor II protein (pET -28a-SUMO-blood coagulation factor II ...
Embodiment 3
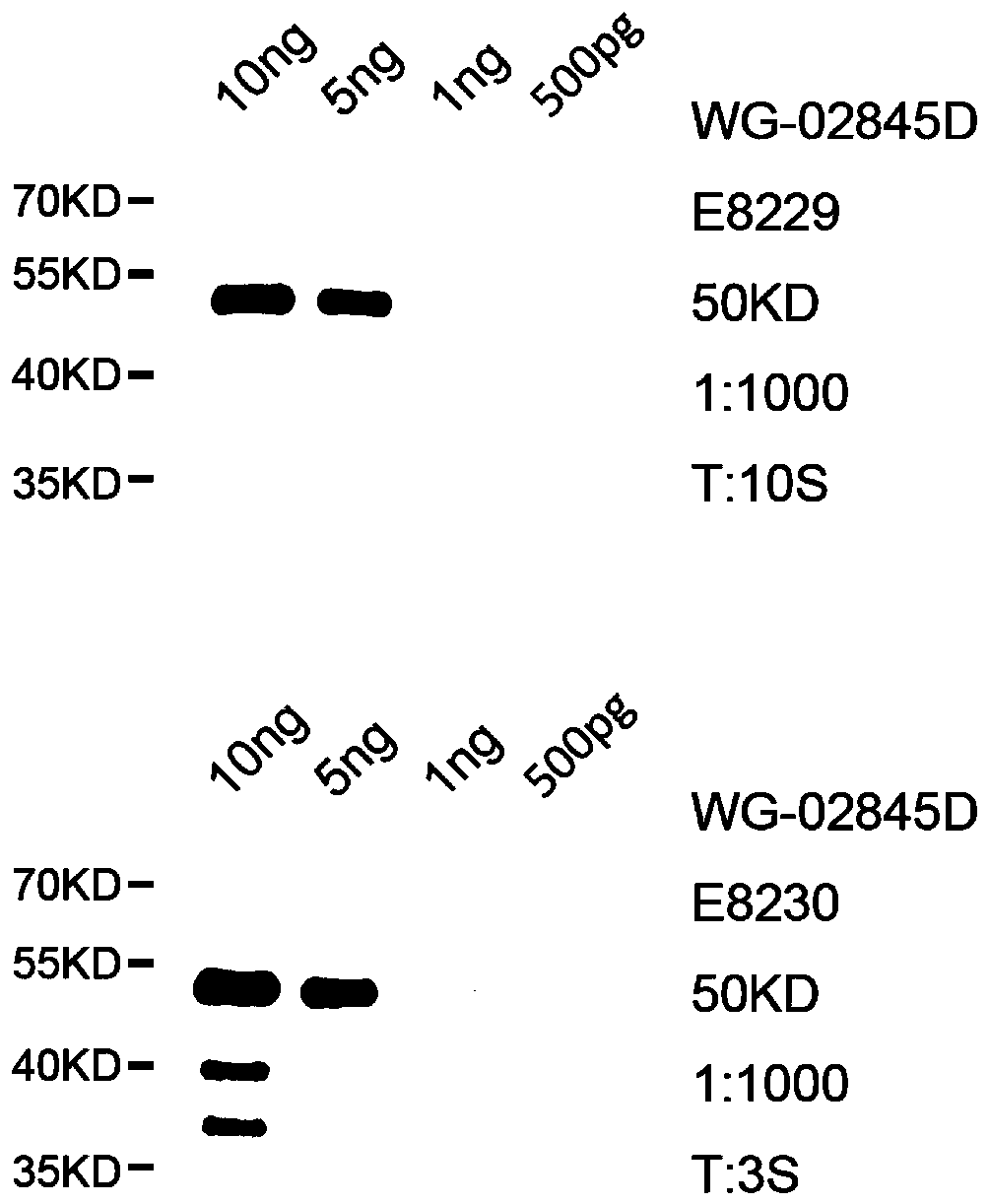
[0031] The acquisition of embodiment 3 polyclonal antibody
[0032] Select healthy 6-week-old experimental-grade Japanese white rabbits (provided by Wuhan Aibotec Biotechnology Co., Ltd.), and collect 10ml of blood from the ear vein for the first time after one week of stability as a negative control, and then use the prepared pET-28a-SUMO- Coagulation factor II protein was used as an antigen to immunize two experimental-grade Japanese white rabbits for multiple times: the immunization site was 4 parts on the back, each site was injected with 250 μl, the immune dose used for the first immunization was 0.3 mg, and complete Freund’s adjuvant was used. 12 days after the second immunization, the dose of antigen used was 0.15 mg, using incomplete Freund's adjuvant; the third immunization was performed 26 days after the first immunization, the dose of antigen used was 0.15 mg, and incomplete Freund's adjuvant was used. Freund's adjuvant; the fourth immunization was carried out 40 da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com