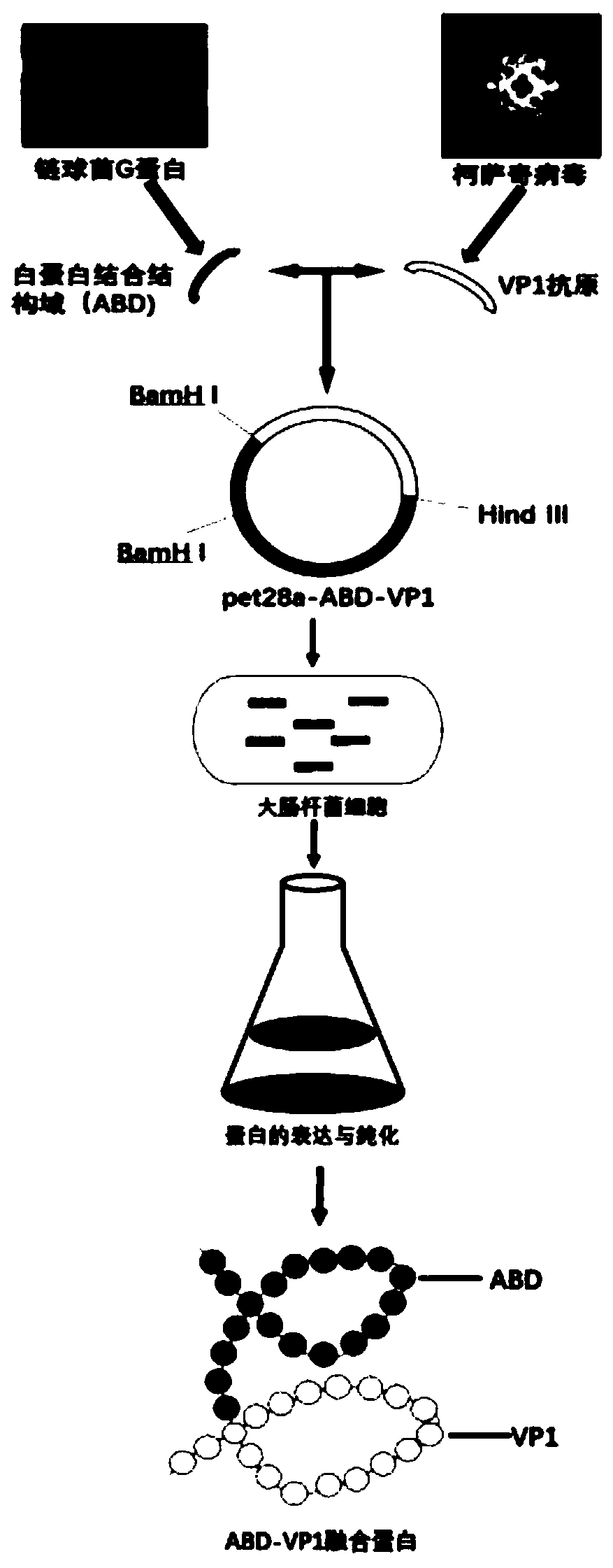
CVB3 myocarditis recombinant protein vaccine targeting draining lymph node and preparation method thereof
A recombinant protein and lymph node technology, applied in the field of genetic engineering, can solve the problems of difficult enrichment and presentation, easy degradation, etc., and achieve the effect of preventing viral myocarditis and improving the effect of immune protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of ABD-VP1 recombinant protein vaccine
[0040] (1) Construction of prokaryotic expression plasmid encoding CVB3 VP1
[0041] Using Coxsackievirus type B3 Nancy strain DNA as a template, use the VP1 upstream primer shown in SEQ ID NO: 7 and the VP1 downstream primer shown in SEQ ID NO: 8 to amplify the full-length coding gene of CVB3 dominant antigen VP1 by PCR method (The amino acid sequence is shown in SEQ ID NO.3, and the nucleotide sequence is shown in SEQ ID NO.4);
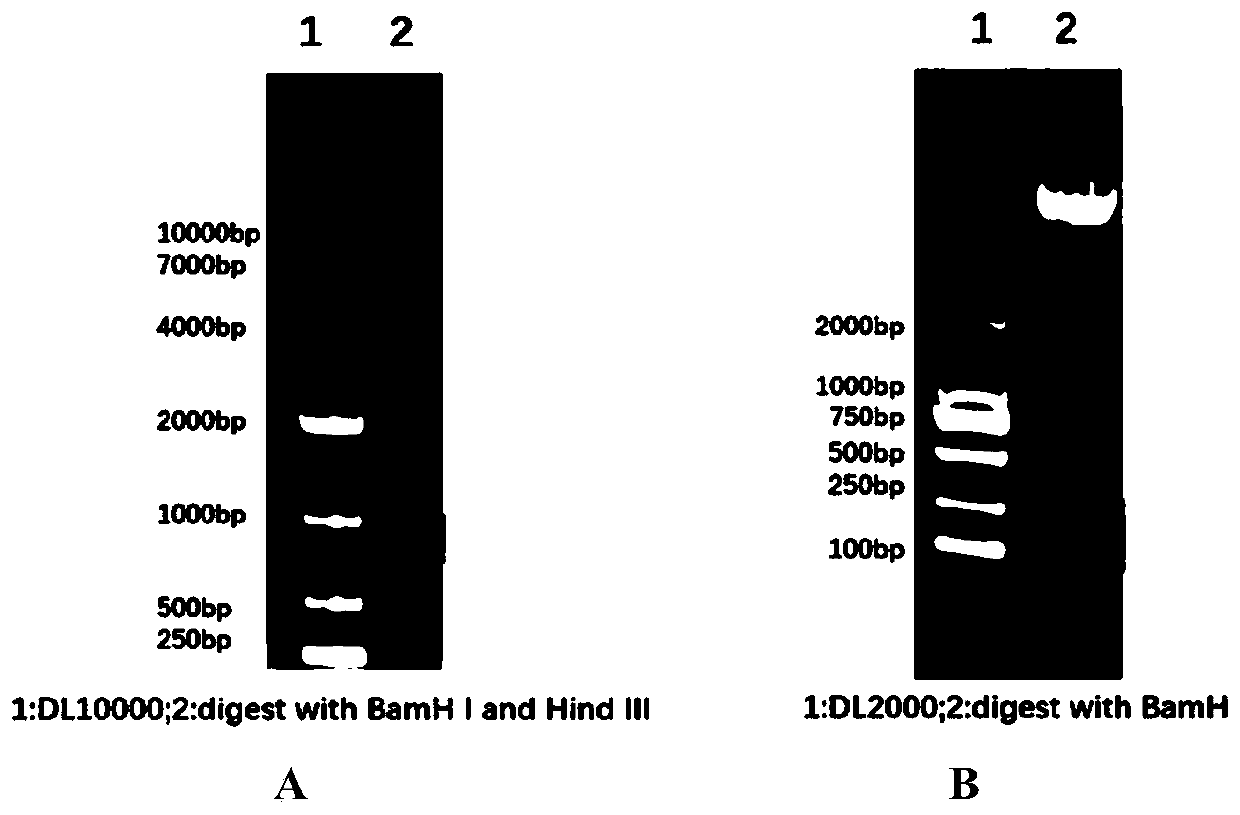
[0042] The target gene was digested by Hind III and BamH I and connected to the pET28a vector which was also digested by the same double enzymes to obtain the prokaryotic expression plasmid (pET28a-VP1) encoding CVB3 VP1;
[0043] (2) Construct the prokaryotic expression plasmid encoding the ABD-VP1 protein vaccine
[0044] The full-length gene encoding ABD was chemically synthesized and constructed into plasmid pUC57 to obtain pUC57-ABD plasmid. Using this plasmid as a tem...
Embodiment 2
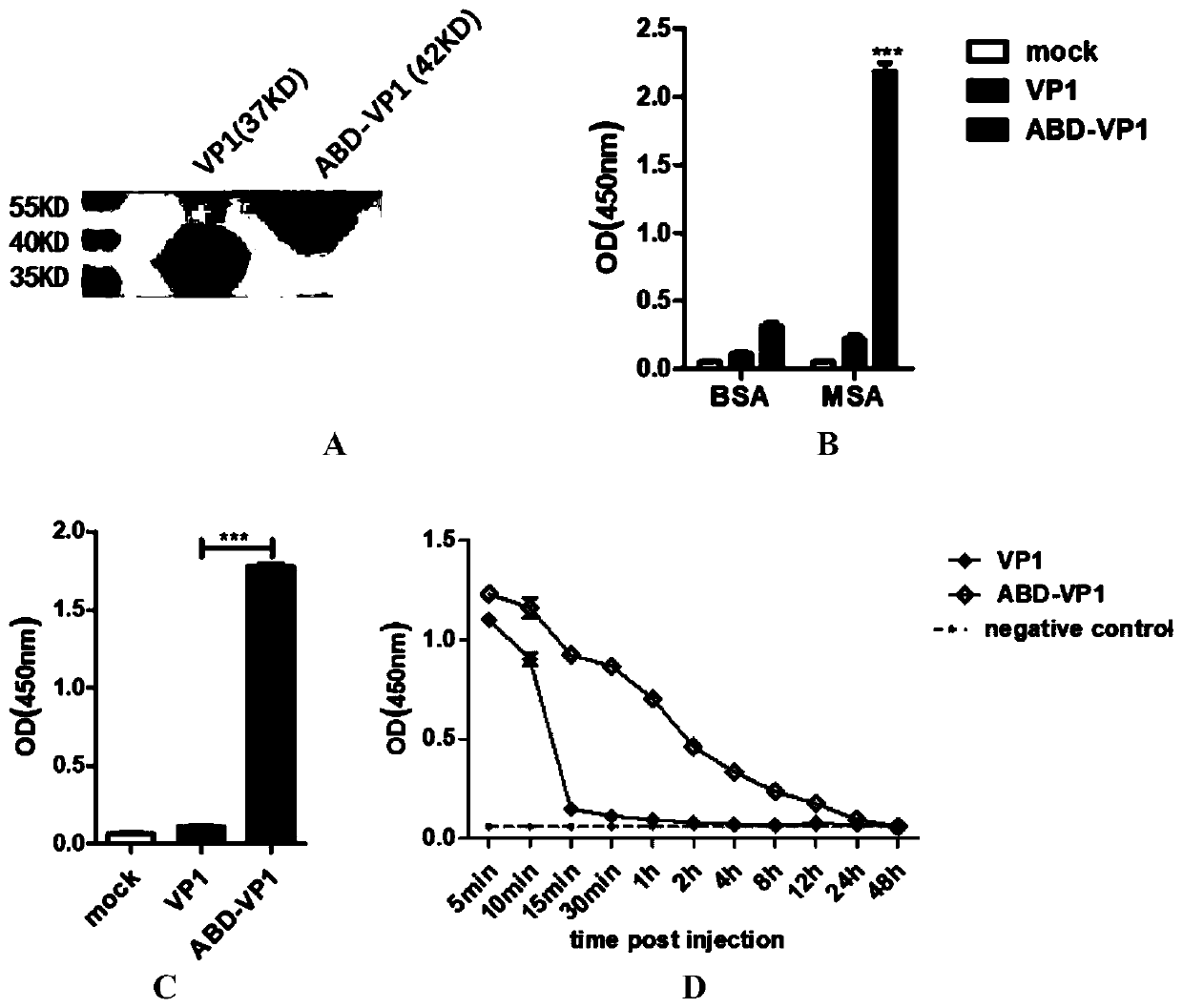
[0054] Embodiment 2: Characteristic detection of ABD-VP1 recombinant protein vaccine
[0055] 1) Binding test of ABD-VP1 and mouse serum albumin (MSA)
[0056] After protein purification, we tested the binding ability of the two proteins to MSA by ELISA method. The specific steps are as follows: first, coat the plate with mouse serum albumin (MSA) and bovine serum albumin (BSA), add 10 μg / ml, 100 μl / hole into a 96-well Elisa plate, overnight at 4°C; wash with 0.05% PBST After three times of blocking with 5% milk, 100 μl / well, incubate at room temperature for 1 h; wash three times with 0.05% PBST, add ABD-VP1 / VP1 (1 μg / ml) to the corresponding well, 100 μl / well, incubate at 37°C for 2 h; 0.05% PBST Wash three times with PBST, add VP1 antibody (1:8,000 dilution), 100μl / well, incubate at room temperature for 1h; wash three times with 0.05% PBST, add HRP-goat anti-rabbit IgG antibody (1:6,000 dilution), 100μl / well, incubate at room temperature 1h; wash five times with 0.05% PBST...
Embodiment 3
[0061] Embodiment 3: ABD-VP1 recombinant protein vaccine subcutaneously immunizes BALB / c mice
[0062] BALB / c male mice aged 6-8 weeks were divided into 3 groups: PBS, VP1, ABD-VP1, 6 mice in each group. A total of three immunizations were performed, and blood was collected from the posterior canthus vein every other week. The serum was collected at 37°C for 30 minutes and centrifuged at 3,000 rpm for 30 minutes. The serum was collected and frozen at -80°C for subsequent testing.
[0063] Immunization methods are as follows:
[0064] 1) Draw 25 μg recombinant protein (dissolved in 200 μl PBS) with a syringe and directly inject it into the subcutaneous part of the mouse,
[0065] 2) Take blood after one week,
[0066] 3) Repeat the immunization process of 1)+2) two weeks later,
[0067] 4) A total of 3 times of immunization, the total amount of VP1 protein is about 50-100 μg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com