Temozolomide polymer prodrug and preparation method and application thereof
A technology of temozolomide and polymers, which is applied in the field of temozolomide polymer prodrugs and its preparation, can solve the problems of entrapped drug leakage, sudden release, and high specificity of the carrier, and solve the problems of easy degradation, difficult dissociation, and overcoming easily leaked effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
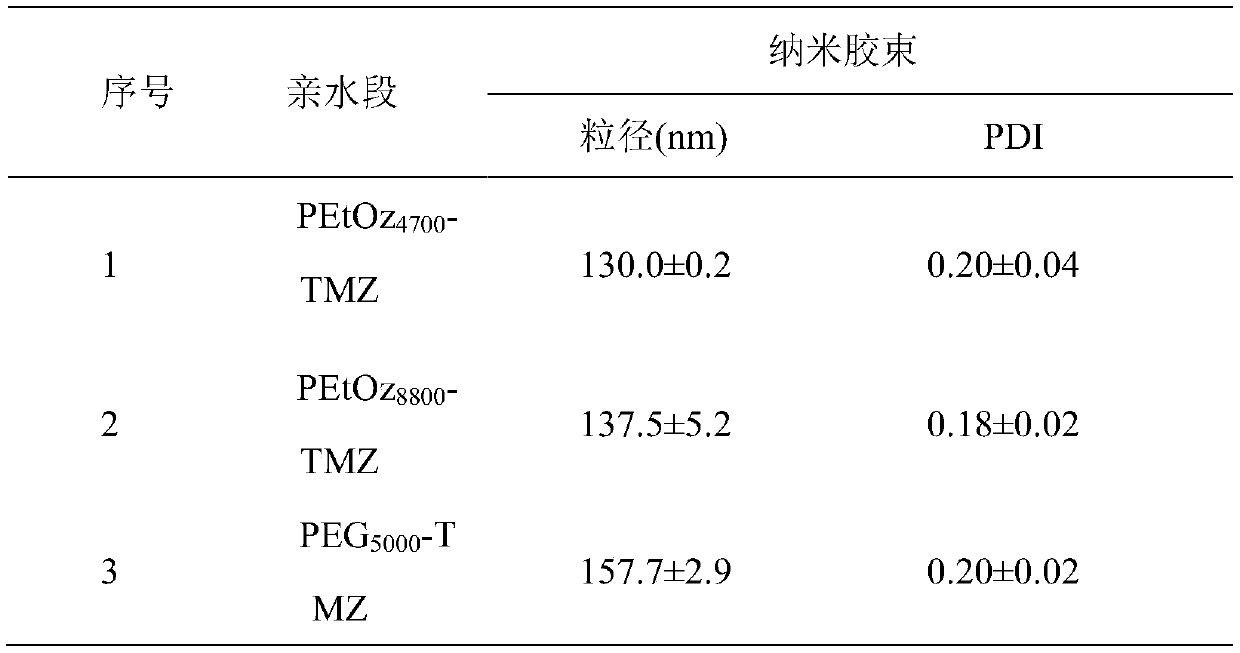
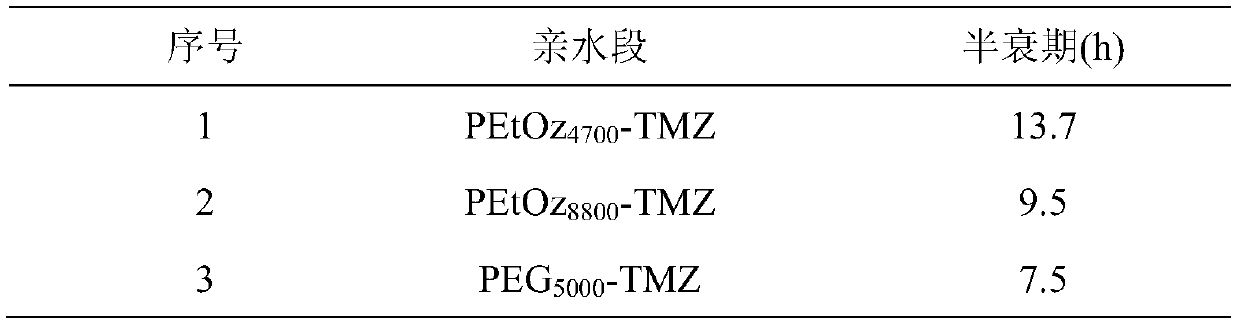
[0021] Embodiment 1 synthetic target molecular weight is poly(2-ethyl-2-oxazoline) (PEtOz-OH) of 5000Da
[0022] Poly(2-ethyl-2-oxazoline) (PEtOz-OH) was synthesized by ring-opening polymerization of EtOz as monomer under the initiation of methyl p-toluenesulfonate (MeOTs). First, under nitrogen protection, EtOz (10.0g, 0.101mol), acetonitrile (33.3ml) and methyl p-toluenesulfonate (0.34g, 1.83×10 -3 mol) into the reaction flask. Then react at 100°C for 24h. Then it was cooled to room temperature and 18 ml of 0.1M potassium hydroxide methanol solution was added, the reaction was terminated after stirring for 4 hours, and then settled with glacial ether, the solid was collected and dried in vacuum for 24 hours. The actual molecular weight was measured to be 4700 Da.
Embodiment 2
[0023] Embodiment 2 Synthesis target molecular weight is poly(2-ethyl-2-oxazoline) (PEtOz-OH) of 7500Da
[0024] First, under nitrogen protection, EtOz (10.0g, 0.101mol), acetonitrile (33.3ml) and methyl p-toluenesulfonate (0.24g, 1.32×10 -3 mol) into the reaction flask. Then react at 100°C for 24h. Then it was cooled to room temperature and methanol solution of potassium hydroxide (0.1M, 33ml) was added, the reaction was terminated after stirring for 4h, and then settled with glacial ether, the solid was collected and dried in vacuum for 24h. The actual molecular weight was measured to be 8800 Da.
Embodiment 3
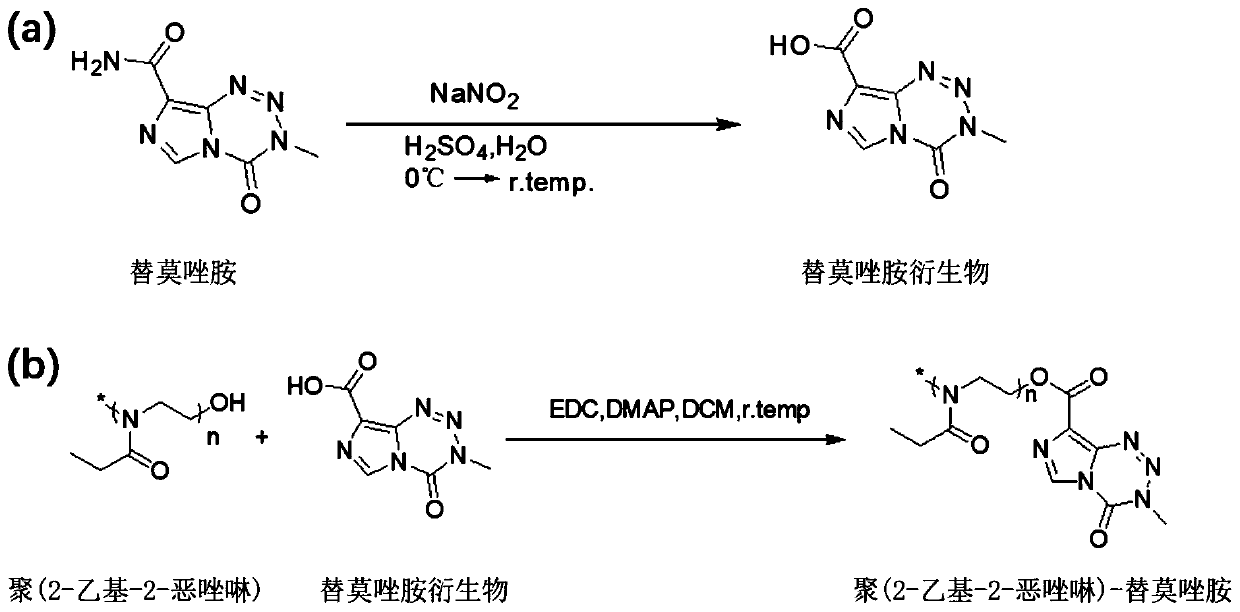
[0025] Example 3 Synthesis of carboxy-terminal temozolomide derivatives (TMZ-COOH)
[0026] Such as figure 1 As shown in a, 5 mg of TMZ was dissolved in 47 ml of concentrated sulfuric acid, the resulting yellow solution was cooled to 0°C under nitrogen, and 47 ml of an aqueous solution of sodium nitrite (4.02 g, 72.8 mmol) was added dropwise. After warming the mixture to room temperature while stirring in the dark for 24 h, the mixture was cooled to 0 °C and quenched with 122 g of ice. Further stirring at 0 °C resulted in the precipitation of a white solid, which was isolated by vacuum filtration. Washed with cold water and dried under vacuum to give TMZ-COOH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com