Polyarylether polymer containing photoelectric functional groups, preparation method and application thereof
An optoelectronic function, polyarylene ether technology, applied in the field of preparation, polyarylene ether polymers, can solve problems such as little research, achieve controllable degree of polymerization, good thermal stability and solubility, good photoelectric activity and photoelectricity The effect of functional characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
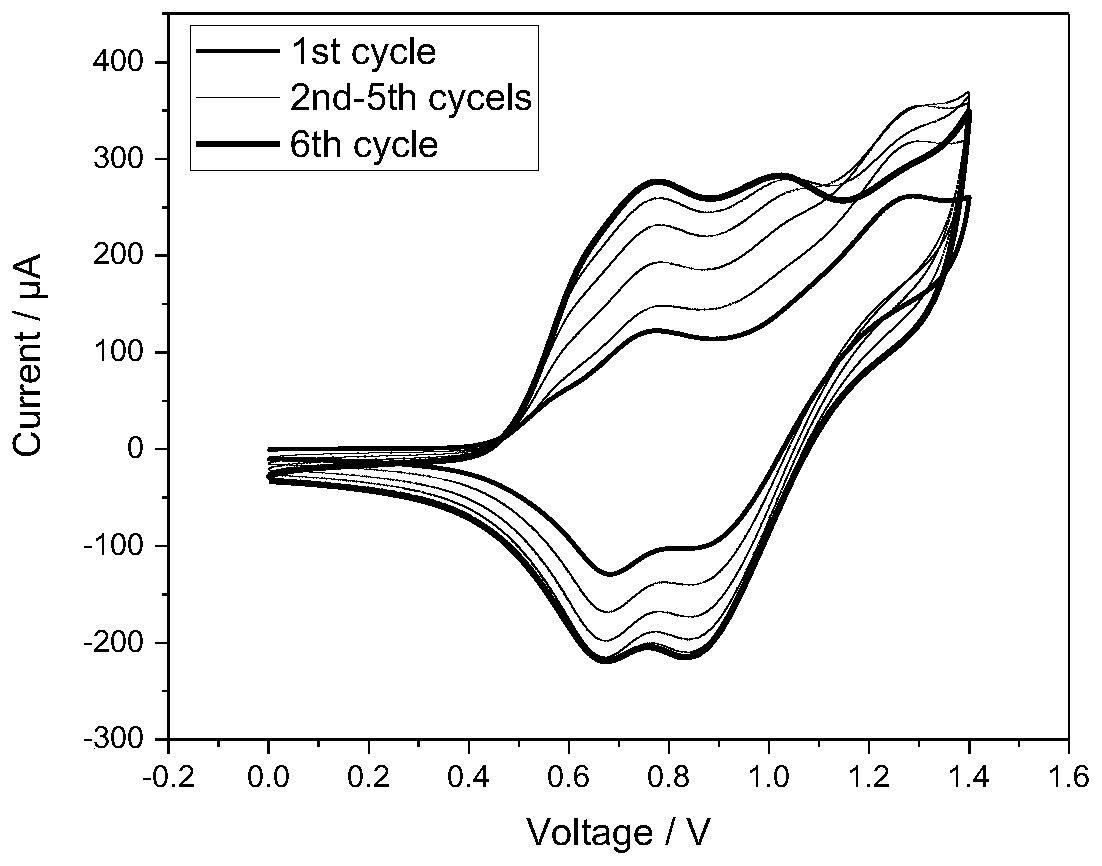
Image
Examples
Embodiment 1
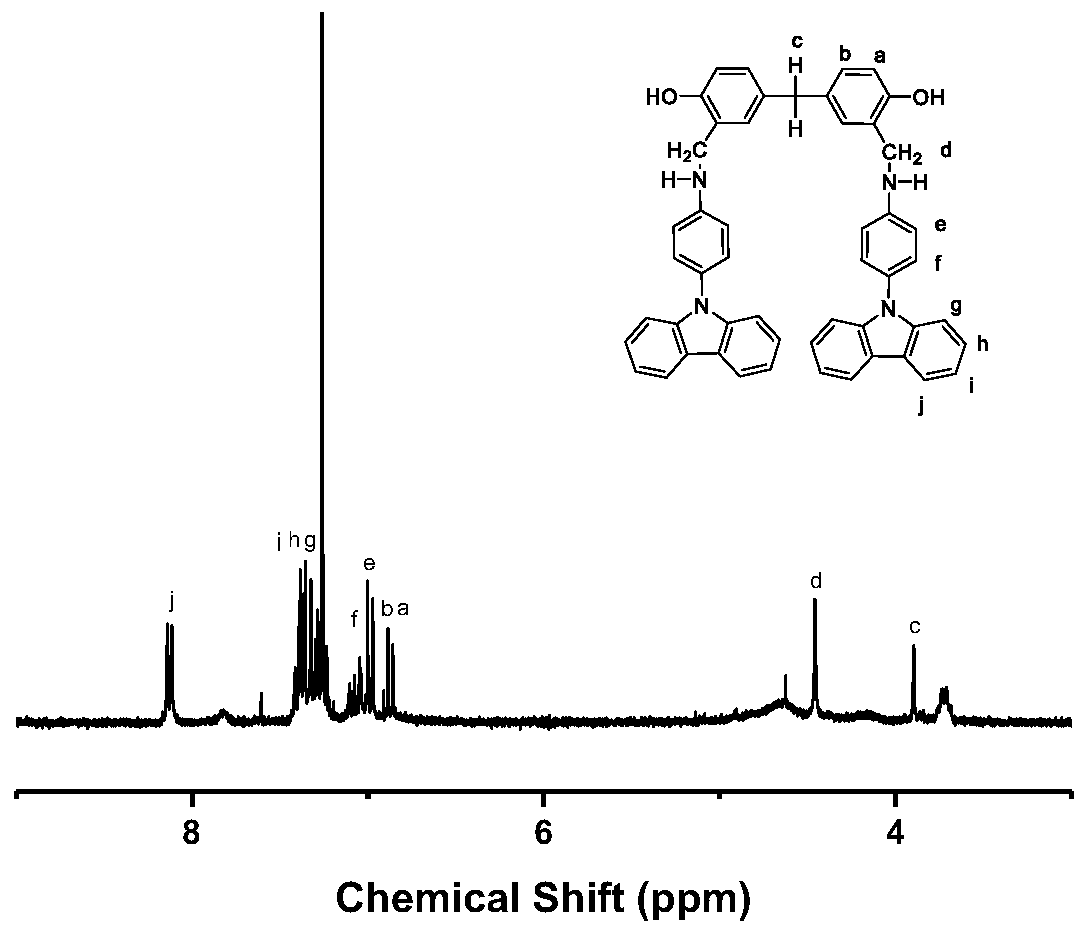
[0067] Synthesis of monomer
[0068] 1) Monomer M1:
[0069] Synthesize monomer M1 according to the route shown in the following formula, and the specific process includes:
[0070]
[0071] Choose a 100mL three-necked flask, connect it with Ar protective gas, spherical condenser, and ground plug in sequence. Under an argon atmosphere, N-(4-aminophenyl)-carbazole (3.22 g, 12.5 mmol) was dissolved in ethanol, heated slightly to 60° C. and stirred to fully dissolve. After dissolving, when the solution was slightly cooled, 5,5'-methylene-disalicylaldehyde (1.28 g, 5 mmol) was added, stirred and heated under reflux for 8 hours to obtain monomer M1. After the reaction was completed, the product was washed with water and ethanol in an ultrasonic cleaner after suction filtration, and the solid product was collected by suction filtration. The product was recrystallized using a mixed solvent of DMF, ethanol and water. The whole process of recrystallization was carried out under ...
Embodiment 2
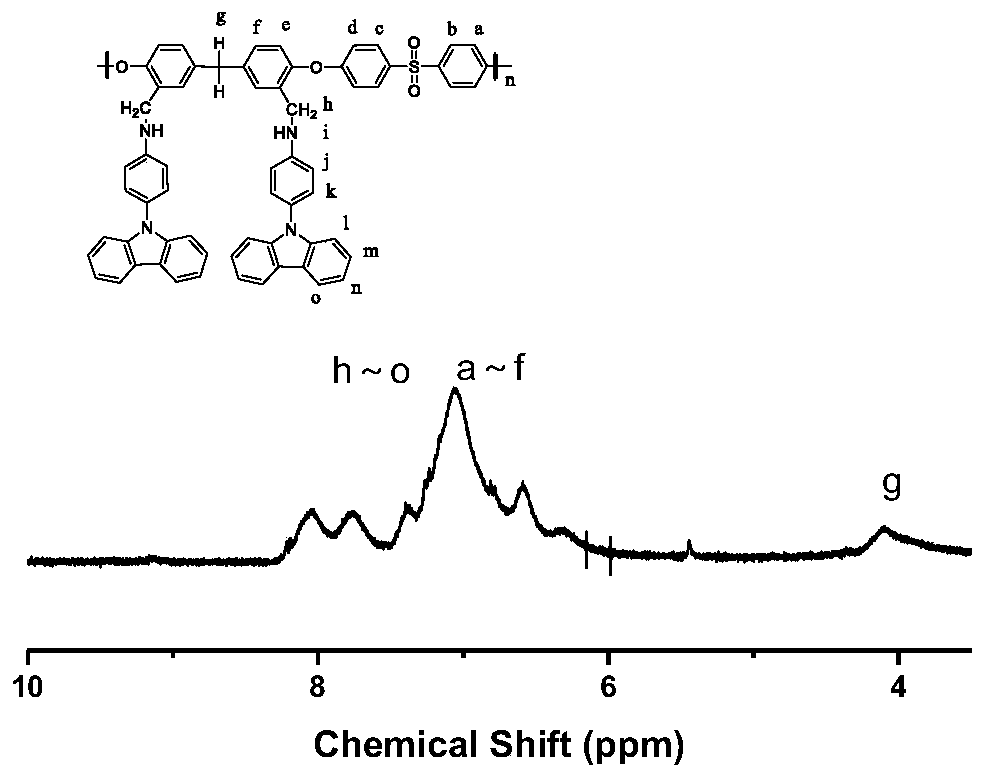
[0084] Prepare P1
[0085] Add monomer M2 (2.82g, 3.00mmol), 4,4-difluorodiphenyl sulfone (0.76g, 3.00mmol), potassium carbonate (0.41g, 3.00mmol), and sulfolane 9mL to the Paddle (mechanical stirring), three-neck flask with condenser tube oil-water separator. The toluene in the oil-water separator is flush with the outlet of the branch pipe. Under the protection of argon, the temperature was controlled at 160°C, and the mixture was heated and stirred. When turbidity appears in the oil-water separator, carry water for 3 hours. When the two-phase interface in the oil-water separator no longer moves, the toluene is distilled off. Then the temperature was raised to 200° C., and the viscosity of the system rose rapidly after 2 hours of reaction. After the reaction was completed, the heating and stirring were stopped, and the mixture in the bottle was poured into distilled water while it was still hot. The off-white strip solid was obtained, and the solid was crushed into powd...
Embodiment 3
[0091] Prepare P2
[0092] Add monomer M 4 (2.73g, 3.00mmol), 4,4'-difluorodiphenyl sulfone (0.76g, 3.01mmol), potassium carbonate (0.39g, 2.86mmol), and sulfolane 9mL to the Fluorine stirring paddle (mechanical stirring) and a three-necked flask with a condenser oil-water separator. The toluene in the oil-water separator is flush with the outlet of the branch pipe. Under the protection of argon, the temperature was controlled at 160°C, and the mixture was heated and stirred. When turbidity appears in the oil-water separator, carry water for 3 hours. When the two-phase interface in the oil-water separator no longer moves, the toluene is distilled off. Then the temperature was raised to 200°C, and after 6 hours of reaction, the viscosity of the system rose rapidly and imploded, and the sample in the reactor was in the shape of jelly. After the reaction is over, stop heating and stirring, and pour the mixture in the bottle into distilled water while it is still hot to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com