Lithium ion battery organic positive electrode material and application thereof
A technology for lithium-ion batteries and cathode materials, applied in the field of preparation of lithium-ion cathode materials, can solve the problems of low output voltage and constraints on the energy density of organic lithium-ion batteries, achieve high redox potential, be conducive to cycle stability, and improve energy effect of density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The synthesis of conjugated aromatic fused ring P-1 is shown below
[0054]
[0055] Dissolve 1.6-dibromopyrene in N-methylpyrrolidone (NMP), add anhydrous sodium sulfide, and carry out polycondensation reaction. After the reaction was completed, centrifuged, washed, dried, and Soxhlet extracted and purified to obtain the conjugated aromatic fused ring derivative polythiopyrene P-1 of the material. The polymer was calculated to be 11 through elemental analysis and halogen content determination.
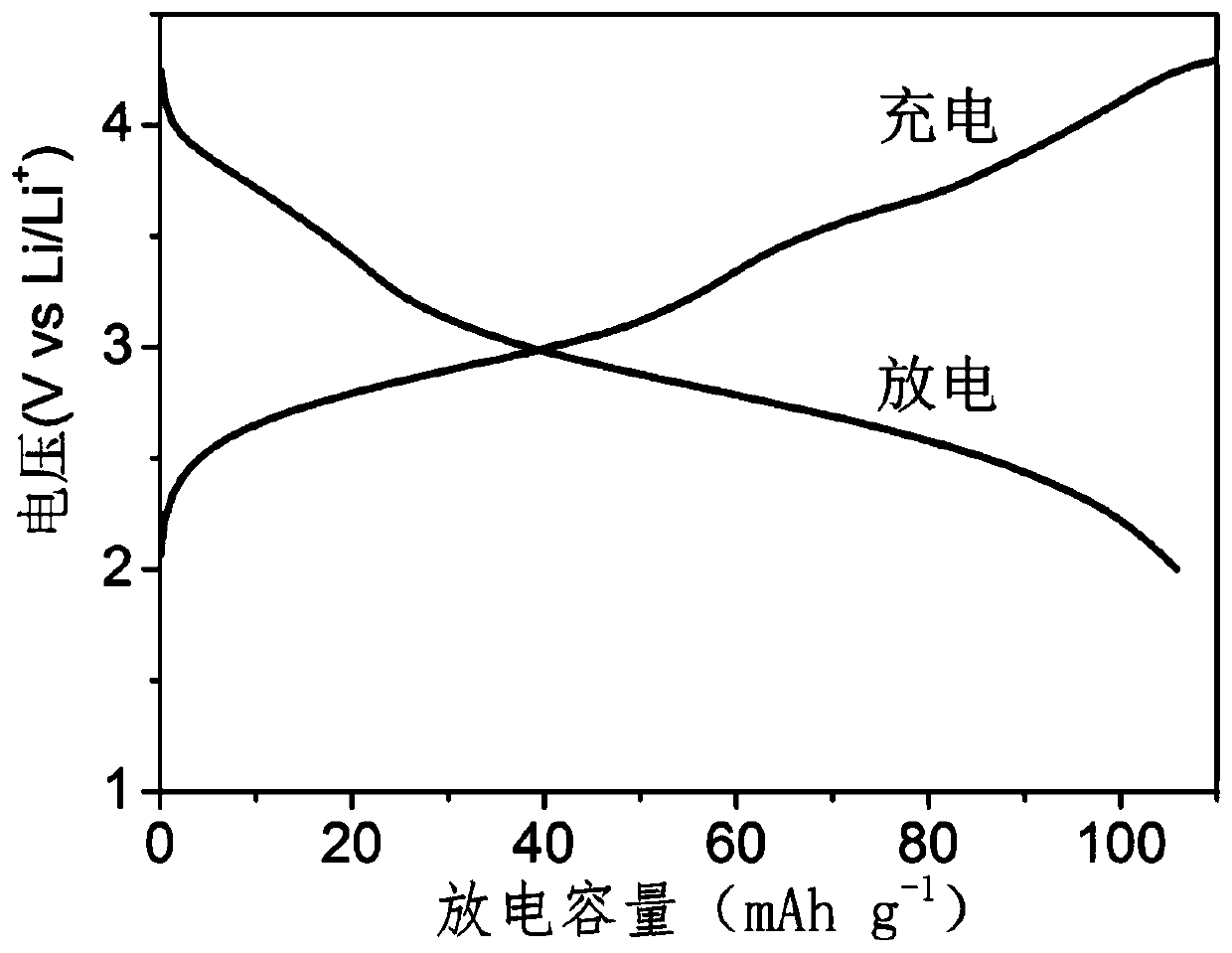
[0056] Mix 30mg of polythiopyrene, 24mg of Super-P and 6mg of polyvinylidene fluoride thoroughly, add 0.5ml of N-methylpyrrolidone, grind again to obtain a homogeneous slurry, evenly coat it on aluminum foil, and then vacuum at 80°C Dry for 12 hours to prepare an electrode film. In a dry argon glove box, use the prepared electrode film as the positive electrode, glass fiber as the separator, 1.0mol / L lithium perchlorate propylene carbonate solution as the electrolyte, and m...
Embodiment 2
[0059] The synthesis of the conjugated aromatic fused ring P-8 is shown below:
[0060]
[0061] Dissolve pyrene diboronic acid ester in a mixed solution of dioxane and water (volume ratio, 1:1), add an equivalent amount of 2,5-dibromothiophene, add a palladium catalyst, and heat up for 24 hours after deoxygenation . After the reaction is completed, the reaction solution is poured into methanol, the precipitate is precipitated, centrifuged, washed, and purified by Soxhlet extraction to obtain the conjugated aromatic fused ring derivative thienylpyrene polymer of the material, and the polymer is between 8- Between 20.
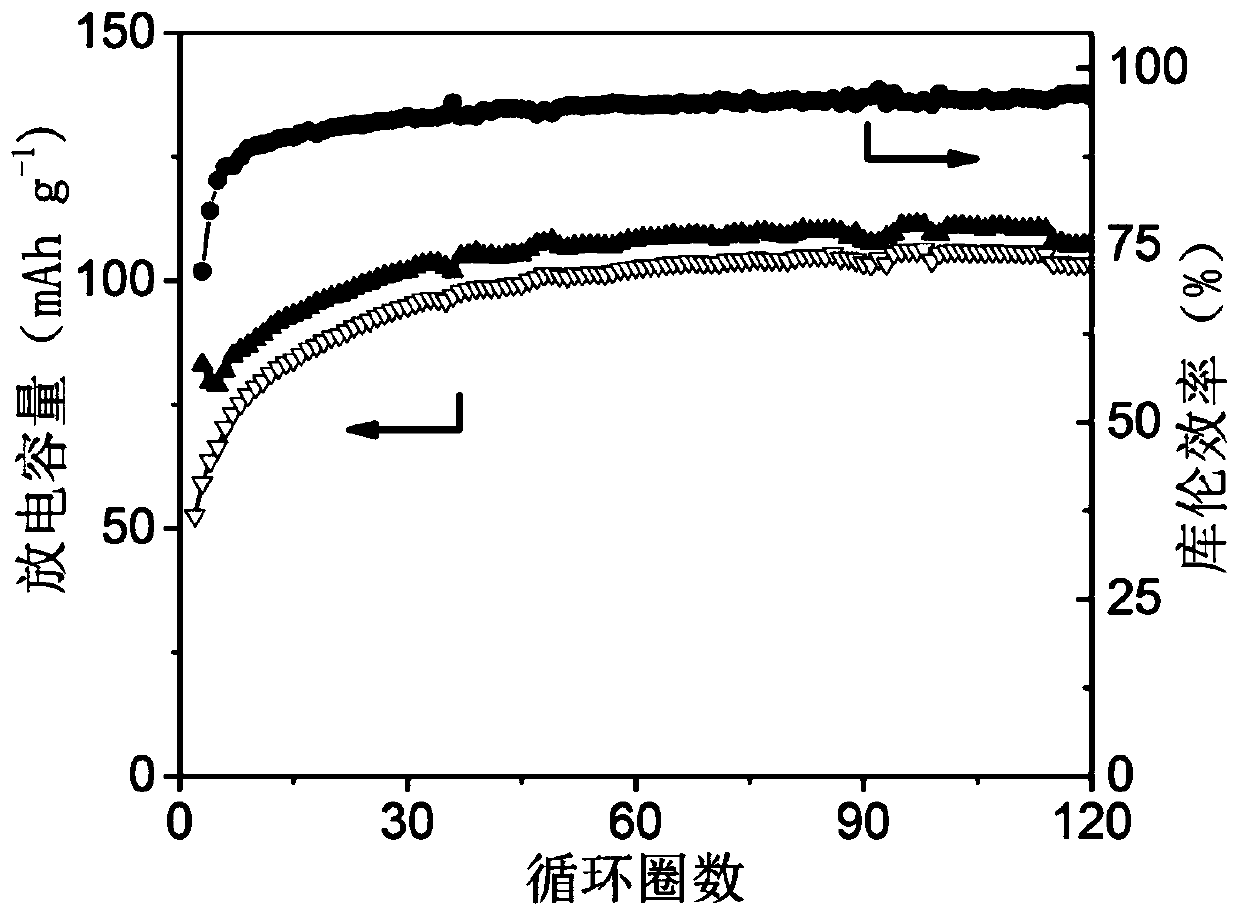
[0062] Mix 30mg of thienylpyrene polymer, 24mg of Super-P and 6mg of polyvinylidene fluoride thoroughly, add 0.5ml of N-methylpyrrolidone, and grind again to obtain a homogeneous slurry, which is evenly coated on aluminum foil, and then at 80 °C for 12 hours in vacuum to prepare an electrode film. In a dry argon glove box, use the prepared electrode film a...
Embodiment 3
[0065] The synthesis of the conjugated aromatic fused ring P-14 is shown below:
[0066]
[0067] Dissolve pyrene diboronic acid ester in the mixed solution of dioxane and water (volume ratio, 1:1), add equivalent 3,7-dibromo-N methylphenothiazine, add palladium catalyst, remove Oxygen post-heating reaction for 24 hours. After the reaction is completed, the reaction solution is poured into methanol, the precipitate is precipitated, centrifuged, washed, and purified by Soxhlet extraction to obtain the conjugated aromatic condensed ring derivative phenothiazine pyrene polymer, the polymer of which is between 8 Between -20.
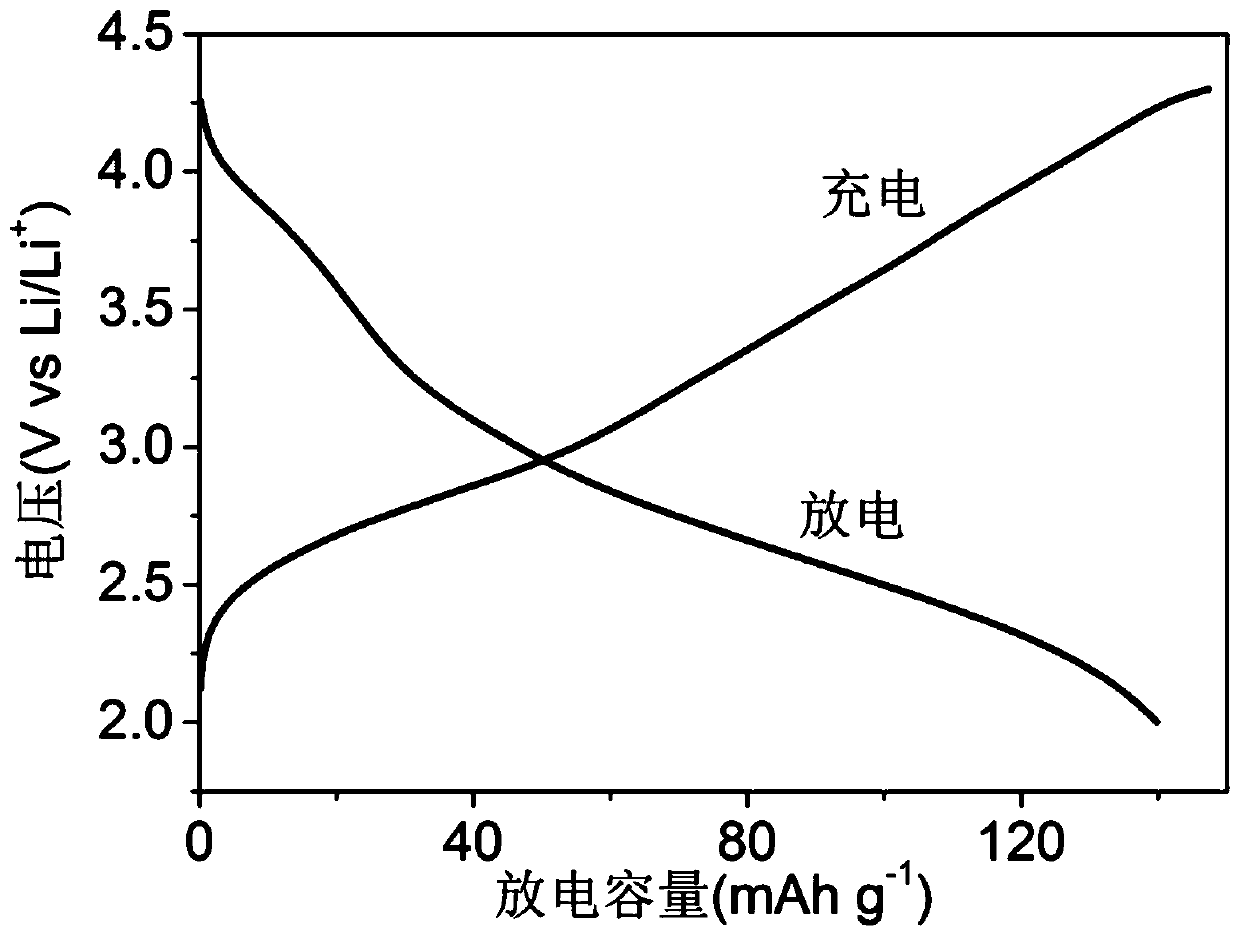
[0068] Mix 30mg of phenothiazine pyrene polymer, 24mg of Super-P and 6mg of polyvinylidene fluoride thoroughly, add 0.5ml of N-methylpyrrolidone, and grind again to obtain a homogeneous slurry, which is evenly coated on aluminum foil, and then placed on Vacuum drying at 80°C for 12 hours to prepare an electrode film. In a dry argon glove box, use the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Energy density | aaaaa | aaaaa |
| Energy density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com