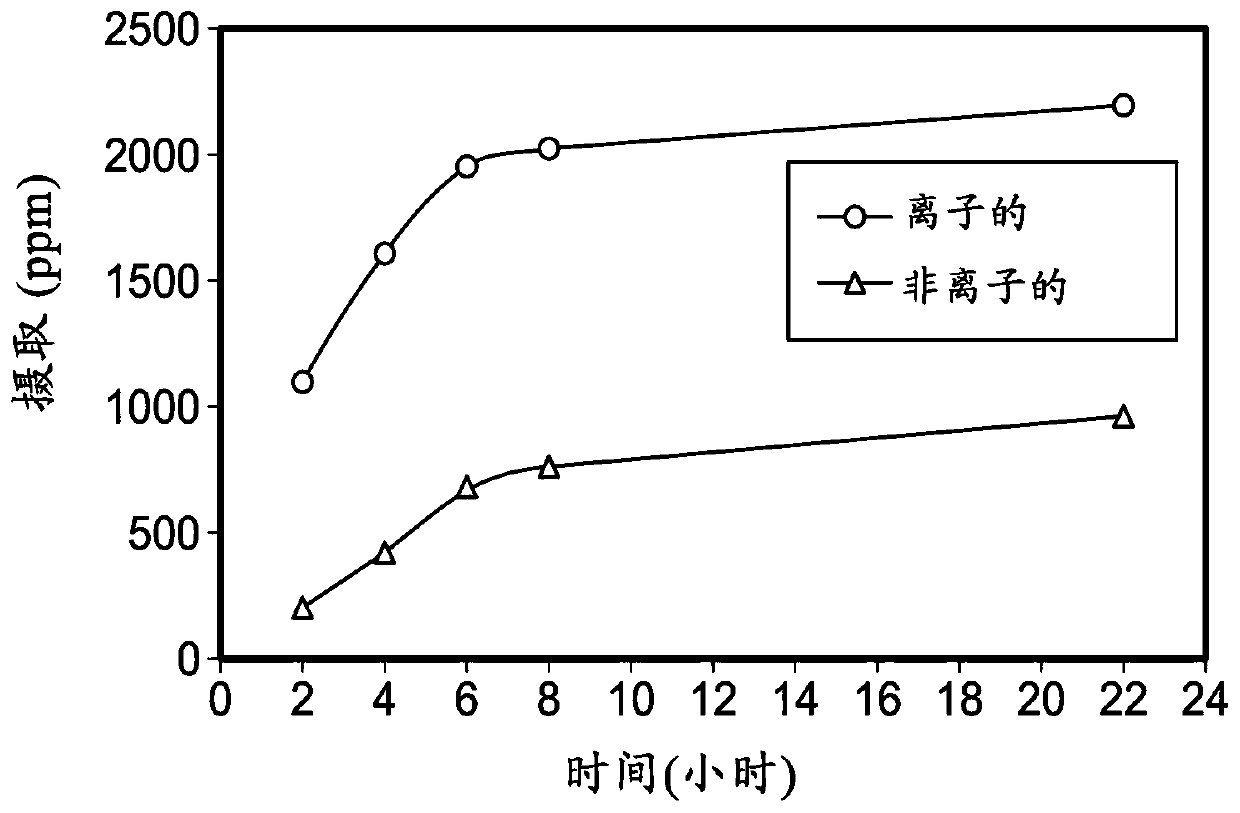
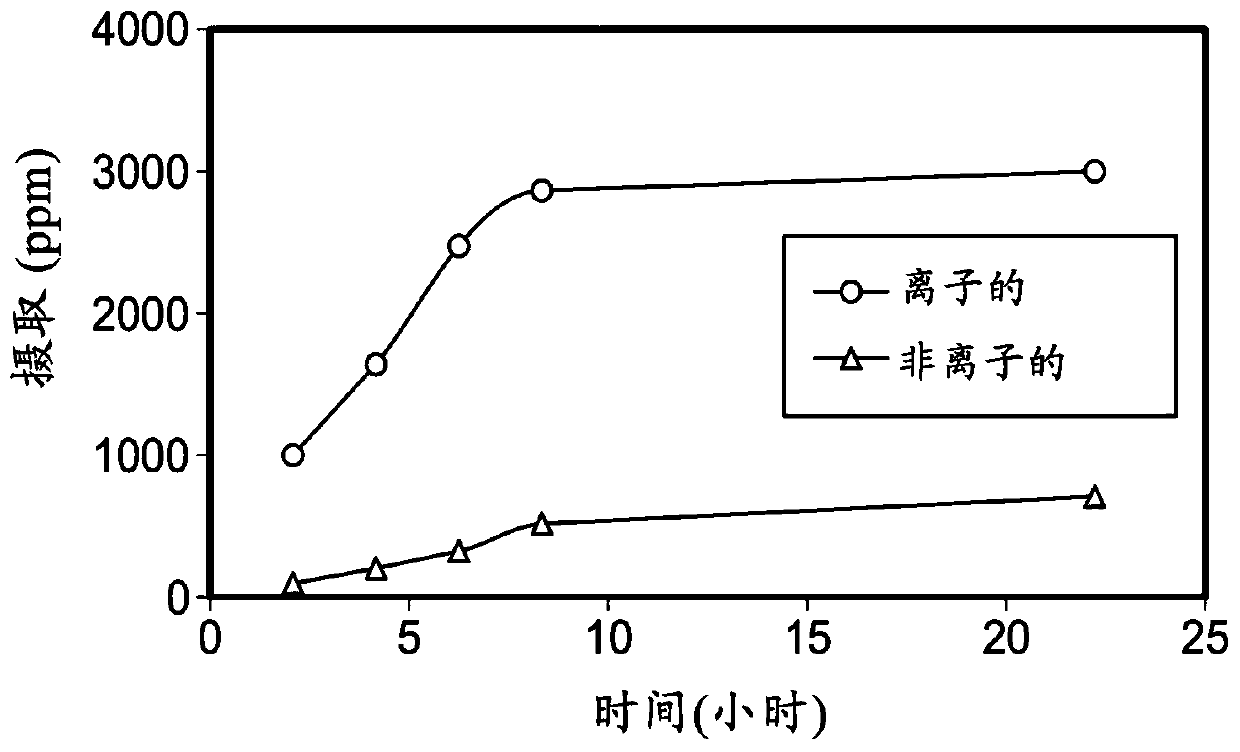
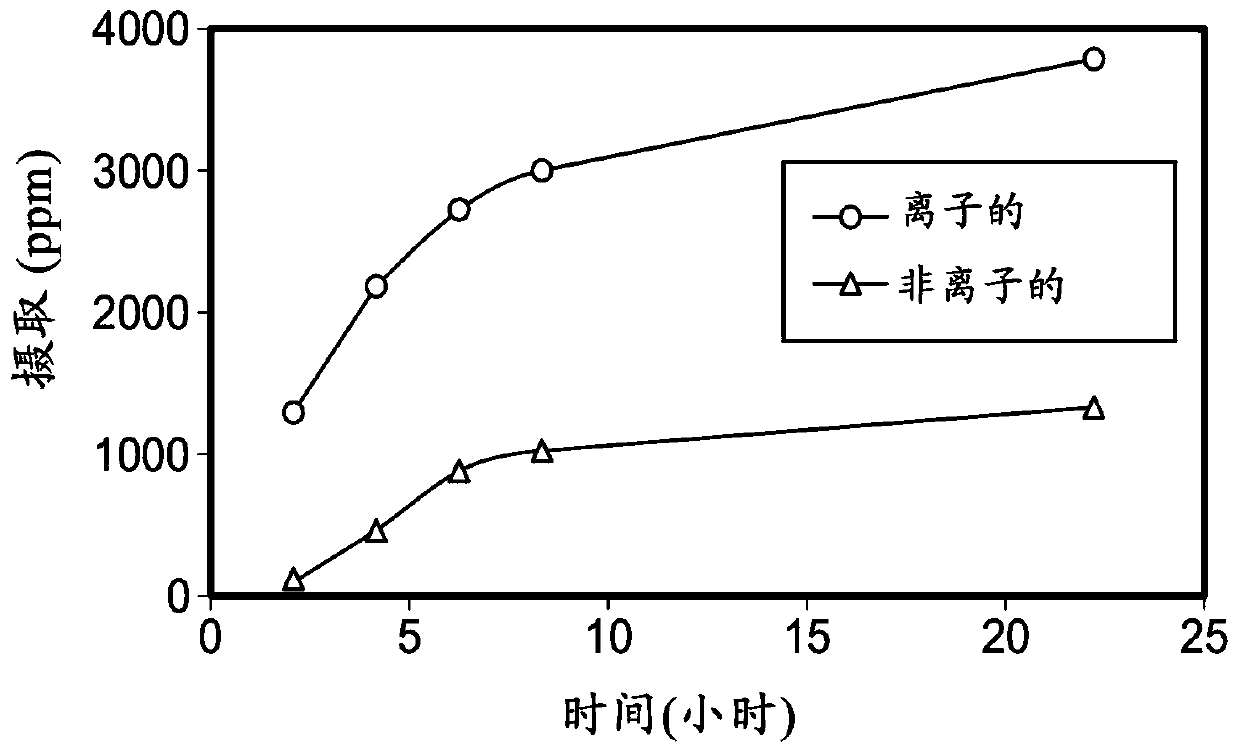
Ionically modified silicones, compositions, and medical devices formed therefrom
An organosilicon compound, the technology of the compound, which is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, instruments, etc., can solve the problems of restricting the curative effect, blurring, long contact time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] Example 1: 4-(2(-Acryloyloxy)-4-(2-(1,1,1,3,5,5,5-heptamethyltrisiloxane-3-yl)ethyl ) cyclohexyl) oxy-4-oxobutyric acid synthesis
[0226] To a round bottom flask equipped with a stir bar, reflux condenser and dropping funnel was added 100 grams of vinylcyclohexyl epoxy functionalized trisiloxane, and toluene, and the reaction mixture was heated to 70-75°C. At this point, catalytic amounts of titanium isopropoxide and TEMPO (2,2,6,6-tetramethylpiperidine 1-oxy) were added and the reaction mixture was further heated to 90°C. To the reaction mixture was gradually added 21 grams of acrylic acid. After completion, the reaction mixture was passed over Dowex-WBA resin to remove unreacted acrylic acid. The product was decolorized using activated carbon and filtered over a bed of diatomaceous earth. The filtrate was vacuum stripped to yield the pale yellow product 5-(2-(1,1,1,3,5,5,5,-heptamethyltrisiloxane-3-yl)ethyl)-2 - Hydroxycyclohexyl acrylate.
[0227] Further 5-(2-...
Embodiment 2
[0228] Example 2: (4-(((2-(acryloyloxy)-5-(2-(1,1,1,3,5,5,5-heptamethyltrisiloxane-3-yl Synthesis of )ethyl)cyclohexyl)oxy)-4-oxobut-2-enoic acid
[0229] 5-(2-(1,1,1,3,5,5,5,-heptamethyltrisiloxane-3-yl)ethyl)-2-hydroxycyclohexylacrylate (50 g), Toluene and maleic anhydride (18 grams) were further added to a round bottom flask equipped with a stir bar, reflux condenser and nitrogen inlet. To this were added triethylamine and hydroquinone as catalyst and free radical scavenger respectively and the reaction mixture was heated to 60-65°C. After completion, the product was vacuum stripped, redissolved in chloroform, and washed with brine solution. The organic phase was passed over Tulsion T66-MP resin to remove traces of triethylamine and decolorized using activated carbon. The organic layer was concentrated after removal of the charcoal to yield a pale yellow viscous product.
Embodiment 3
[0230] Example 3: 3-(((2-(acryloyloxy)-5-(2-(1,1,1,3,5,5,5-heptamethyltrisiloxane-3-yl) Synthesis of ethyl)cyclohexyl)oxy)carbonyl)but-3-enoic acid
[0231] To a round bottom flask equipped with a stir bar, reflux condenser and nitrogen inlet was added 5-(2-(1,1,1,3,5,5,5,-heptamethyltrisiloxane-3-yl) ethyl)-2-hydroxycyclohexyl acrylate (50 g) and toluene. To this were added triethylamine and hydroquinone as catalyst and free radical scavenger respectively and the reaction mixture was heated to 60-65°C. To this was gradually added itaconic anhydride (20 g). After completion, the product was vacuum stripped, redissolved in chloroform, and washed with brine solution. The organic phase was passed over Tulsion T66-MP resin to remove traces of triethylamine and decolorized using activated carbon. The organic layer was concentrated after removal of the charcoal to yield a pale yellow viscous product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com