one for nh 3 Co-mn catalyst for catalytic oxidation, preparation method and application thereof
A catalytic oxidation and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. lower problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] WO 3 -CoMn 2 o 4 Catalyst preparation:
[0031] (1) 50wt% manganese nitrate solution is dispersed in ethanol-water solution (wherein ethanol is 1ml, water is 10ml), stirs at 25 ℃, forms the manganese nitrate solution of 10ml0.2mol / l. Slowly add 10ml of 25wt% concentrated ammonia solution dropwise, and keep stirring for 1h. After fully mixing and reacting, record it as solution A. Dissolve and disperse cobalt nitrate in ethanol-water solution (1ml of ethanol and 10ml of water), stir at 25°C to form 10ml of 0.1mol / l cobalt nitrate solution, which is referred to as solution B. The solution B was slowly added dropwise into the solution A, and the stirring time was continued at 25° C. for 1 h, and the reaction was fully mixed. Then transferred to constant temperature heating equipment, reacted at 180°C for 3h, cooled, and calcined at 200°C in an air atmosphere for 5h, with a heating rate of 2°C / min, to obtain spinel-structured CoMn 2 o 4 , remember Catalyst A.
[0032...
Embodiment 2
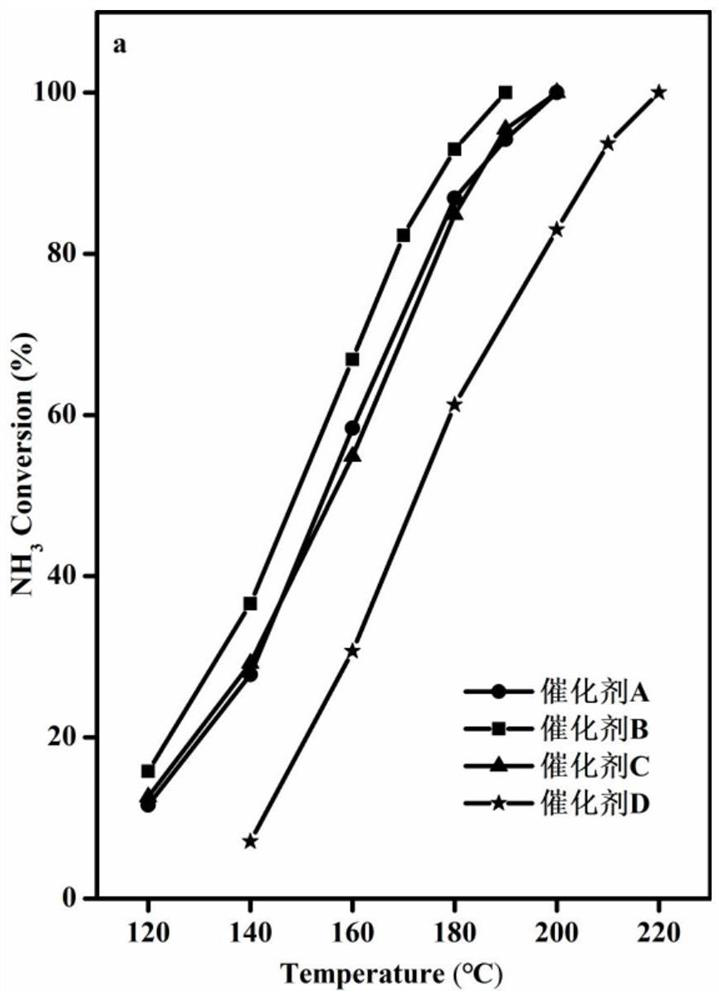
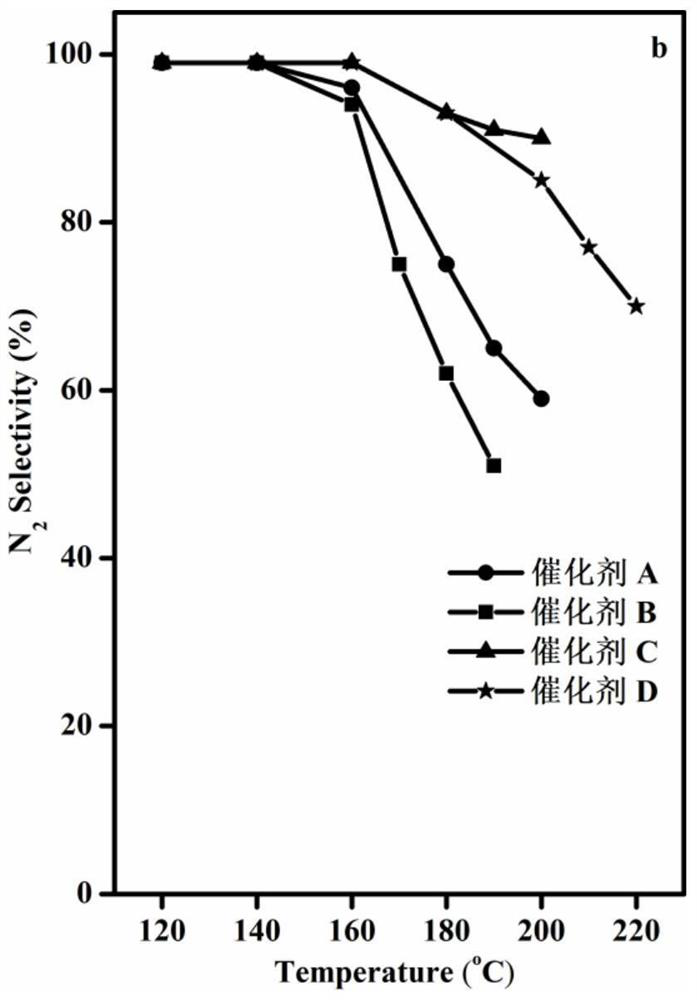
[0043] The WO prepared in Example 1 3 -CoMn 2 o 4 Catalyst and the Co-Mn (CoMn 2 o 4 ), Co-Mn soaked by tetrabutylammonium hydroxide (CoMn 2 o 4 ) and TiO 2 -CoMn 2 o 4 carry out NH 3 Catalytic oxidation, NH 3 Catalytic oxidation performance test is carried out in continuous operation on a fixed-bed reactor, He is used as balance gas, and NH 3 Analyzer and gas chromatograph on-line analyze the gaseous substance NH after the reaction 3 and N 2 .
[0044] The specific reaction conditions are: 1000ppm NH 3 , 10vol%O 2 , He is used as the balance gas, and the reaction space velocity is 40000h -1 , the mass of catalyst is 0.2g. NH 3 Conversion and N 2 The selectivity calculation formula is as follows: NH 3 Conversion %=[(NH before reaction 3 Concentration - NH after reaction 3 Concentration) / NH before reaction 3 Concentration]*100, N 2 Selectivity=[(N after reaction 2 Concentration - N before reaction 2 Concentration) / 500 / (NH before reaction 3 Concentratio...
Embodiment 3
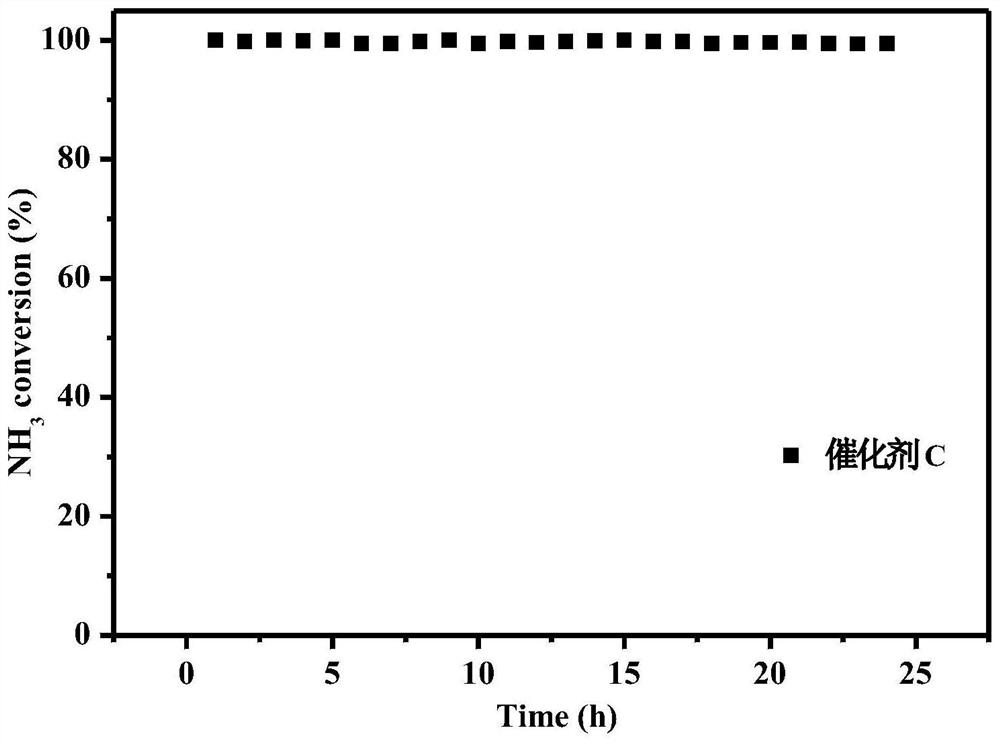
[0046] To the WO prepared in Example 1 3 -CoMn 2 o 4 Catalyst, investigate the stability of this catalyst, keep embodiment 2 reaction conditions, then in 200 ℃ of continuous operation tests the stability performance of catalyst, its stability performance is as figure 2 shown. After 24 hours of stable operation of the catalyst, the NH 3 Catalytic performance and N 2 The selectivity remained unchanged, showing excellent stability performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com