Carbon monoxide donor molecule with fluorescent properties and preparation method and application thereof
A carbon monoxide and donor technology, applied in the direction of organic active ingredients, chemical instruments and methods, medical preparations of non-active ingredients, etc., can solve problems such as poor solubility, toxicity of organic solvents, and limit the application of light-triggered release of carbon monoxide. Biocompatibility, good controllable release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
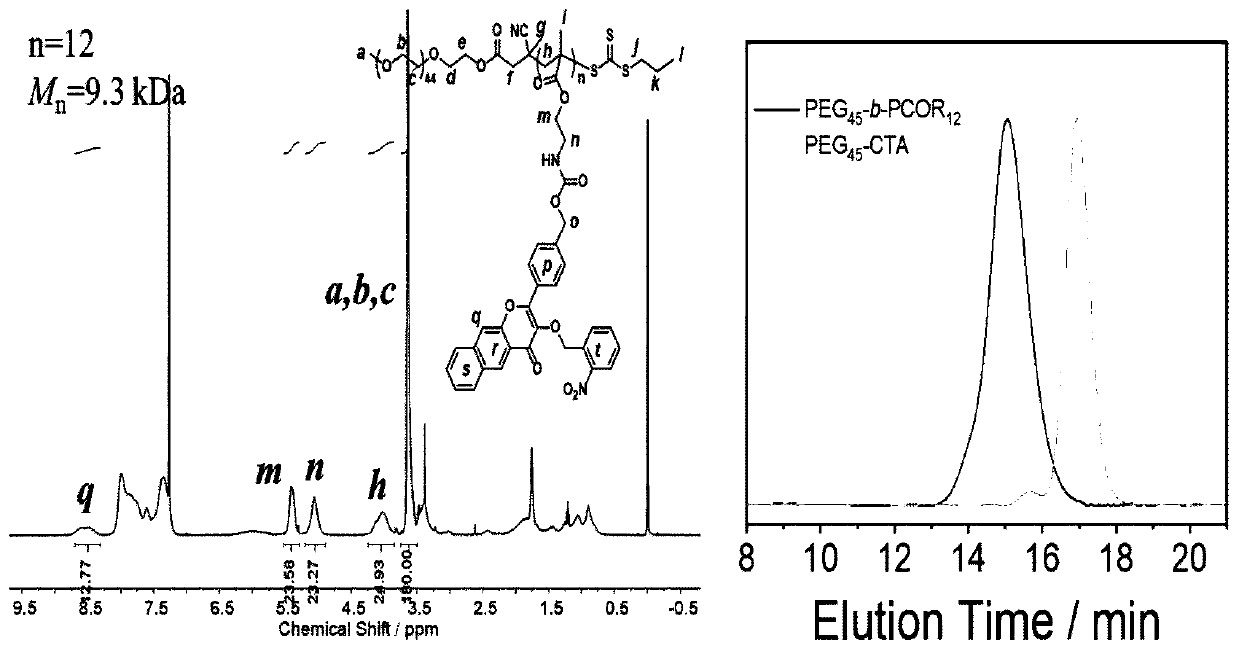
[0028] The present invention also provides a method for preparing a carbon monoxide donor molecule with fluorescent properties, comprising:
[0029] With the compound of formula (II), p-Hydroxybenzaldehyde, R 1 -Br reacts with the compound of formula (III) structure to obtain the compound of formula (I) structure;
[0030]
[0031] where the R 1 C6-C30 arylalkyl containing substituents or C6-C20 alkoxycarbonyl containing substituents;
[0032] R 2 is methyl or hydrogen;
[0033] R 3 is isocyanate or -OCOOH;
[0034] X is oxygen or NH,
[0036] Z is NH or oxygen.
[0037] According to the present invention, the present invention will have the compound of formula (II), p-hydroxybenzaldehyde, R 1 -Br reacts with the compound of the formula (III) structure to obtain the compound of the formula (I) structure; specifically, the steps are as follows: 1) reacting the compound of the formula (II) structure with p-Hydroxybenzaldehyde, after th...
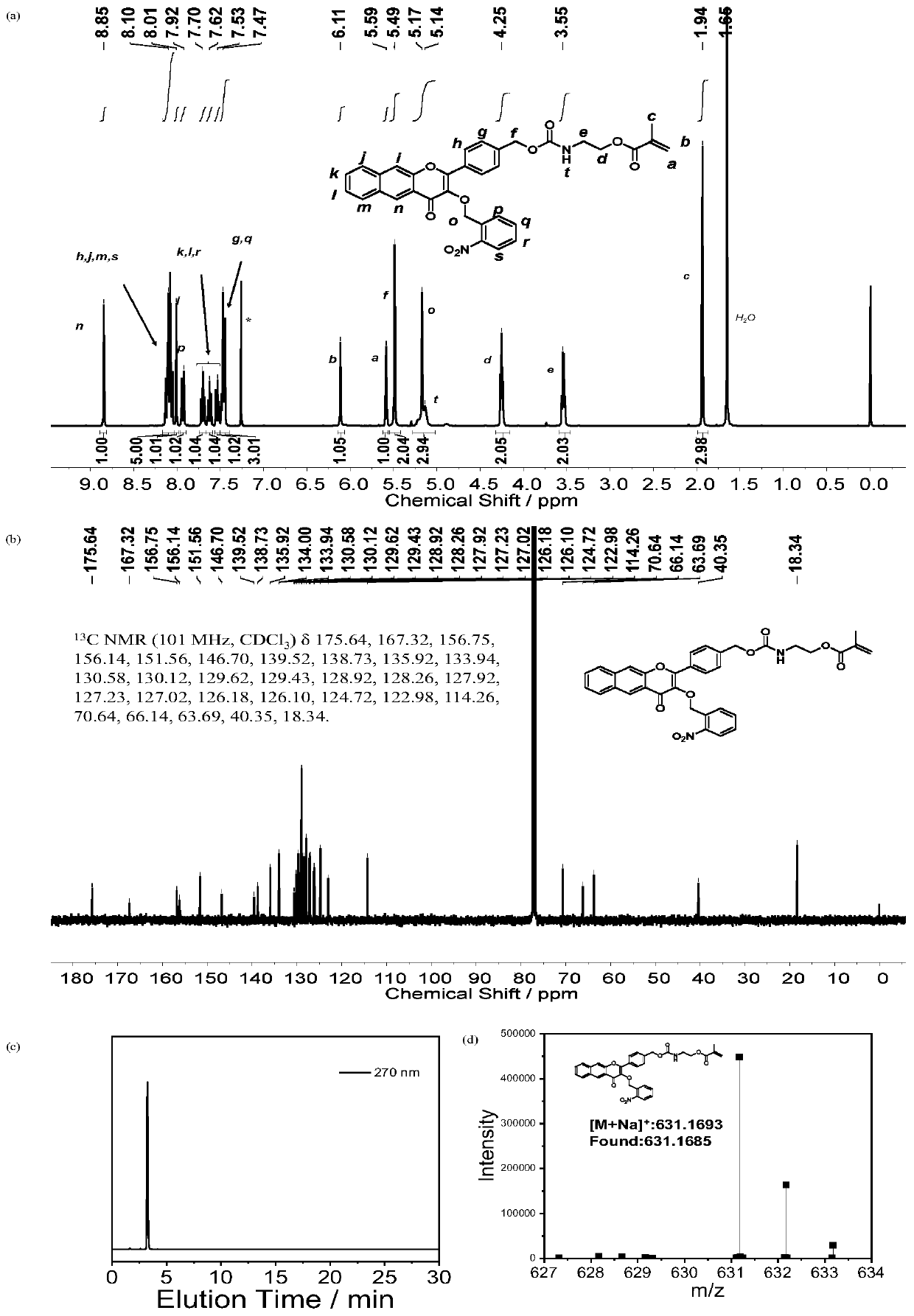
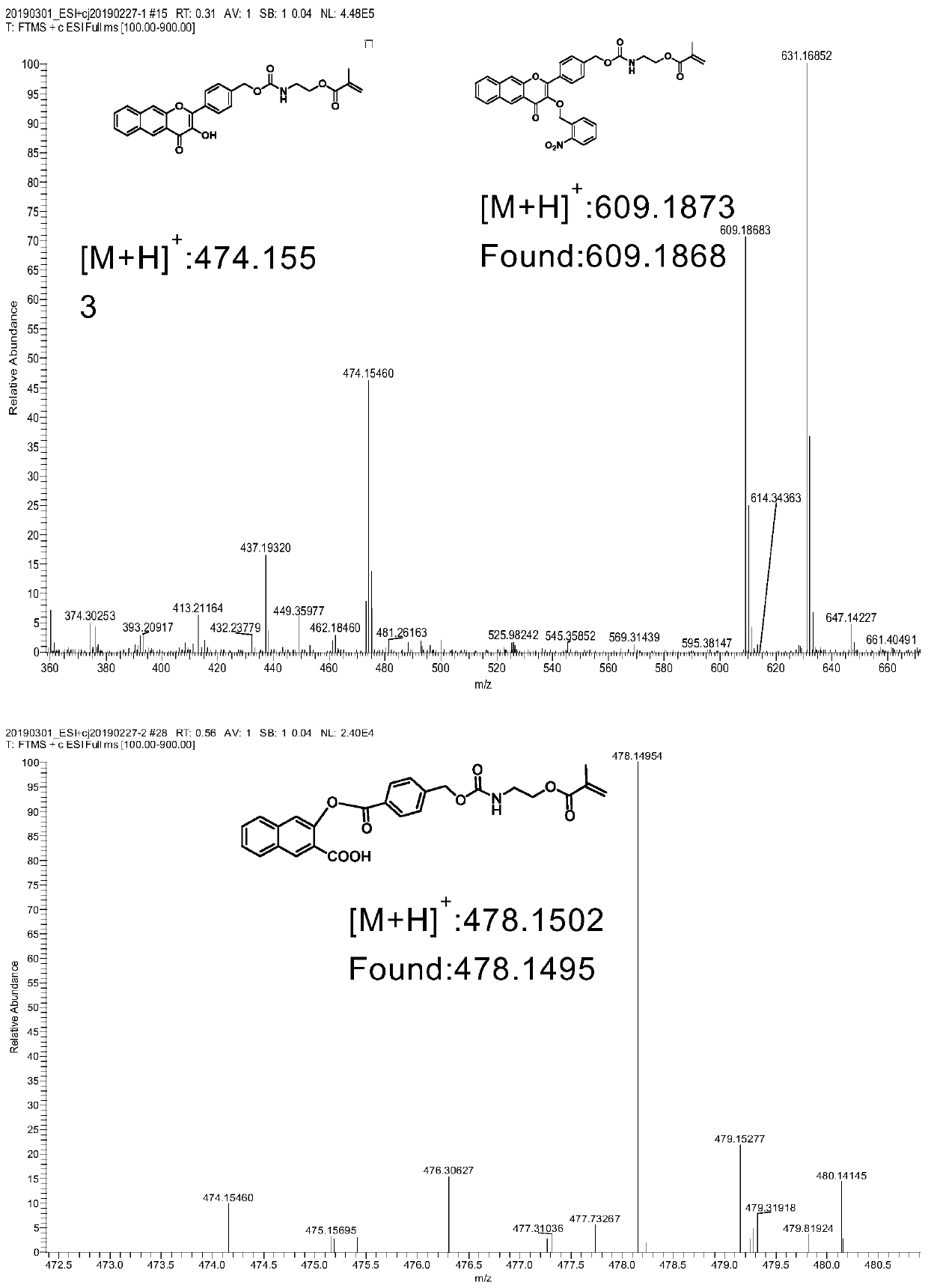
Embodiment 1
[0057] Preparation of a photoresponsive carbon monoxide donor molecule CORM with fluorescent properties (where R is o-nitrobenzyl bromide, X is oxygen, and Y is oxygen)
[0058]
[0059] Preparation method: Weigh 1 (1g, 5.37mmol), add 15mL ethanol to form a suspension, add sodium hydroxide (5.4mL, 5M, 27mmol), stir at room temperature for 30min, p-hydroxymethylbenzaldehyde (748mg, 5.5 mmol) into the reaction flask, stirred at room temperature for 5h, after the reaction was completed, cooled to 0°C, hydrogen peroxide (2.0mL, 30%) was added dropwise into the reaction flask, and stirred overnight; the pH was acidified to 6.5 with 0.5M HCl to form Yellow precipitate, suction filtered, washed with ethanol, and dried to obtain the product, weighed the product (288mg, 1mmol), potassium carbonate (140mg, 1mmol), added 10mL DMF to dissolve for later use, weighed o-nitrobenzyl bromide (258mg, 1.2 mmol) was added to the reaction flask, the mixture was stirred at room temperature for 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com