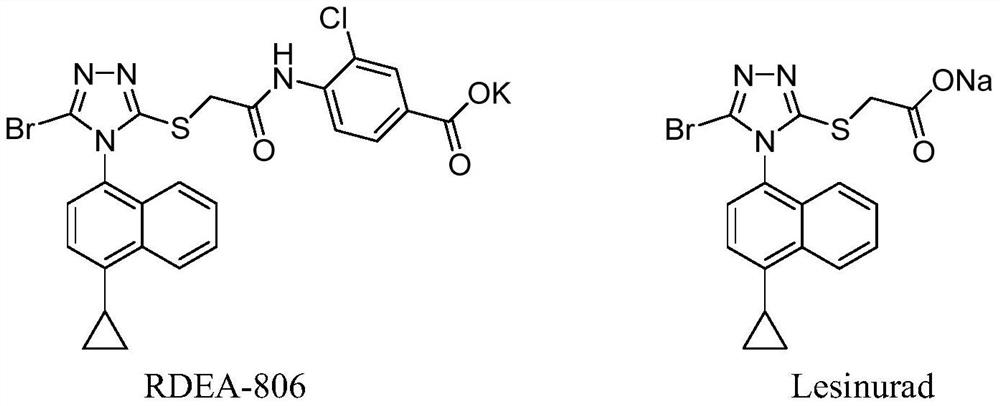
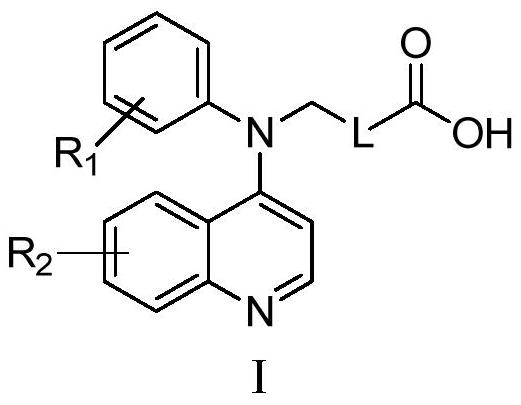
N-phenyl-n-quinoline carboxylic acid compound and its preparation method and medicinal use
The technology of a quinoline carboxylic acid and a compound is applied in the field of drugs related to the treatment of hyperuricemia and gout, and can solve the problems of liver and bone marrow toxicity and allergy, fulminant hepatitis, and limited clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048] Reagents and conditions: a) potassium carbonate, potassium iodide, 80°C; b) palladium acetate, cesium carbonate, 4,5-bisdiphenylphosphine-9,9-dimethylxanthene, 110°C, N 2 ; c) sodium hydroxide, ethanol, 50 ℃
[0049] A. Synthesis of Compound IV-1
[0050] Dissolve compound II-1 (1.1g, 10mmol), compound III-1 (1.5g, 10mmol) in 20mL DMF, add potassium carbonate (2.8g, 20mmol), potassium iodide (332mg, 0.2mmol), react at 85°C for 10h . After the TLC monitoring reaction was complete, most of the DMF was evaporated, ethyl acetate and water were added for extraction, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated for column chromatography separation (petroleum ether-ethyl acetate 10:1-6: 1) to obtain compound IV-1, 1.44 g of light yellow oil, yield: 80%. HR-ESI-MS: m / z=179.0974[M+H]+, calculated for C 10 h 12 FN 2 :179.0979.
[0051] B. Synthesis of Compound VI-1
[0052] To compound IV-1 (128mg, 0.7mmol), compoun...
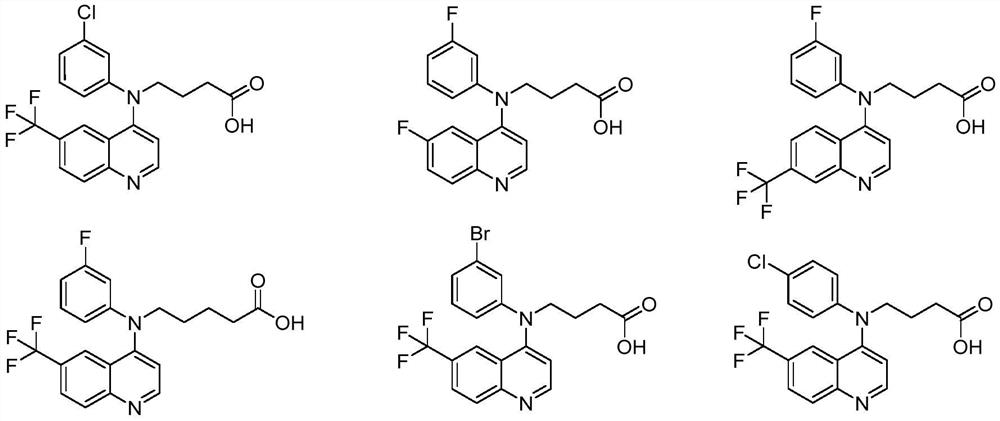
Embodiment 2~19
[0056] Examples 2-19 were prepared according to the synthesis method of Example 1.
Embodiment 2
[0057] Example 2: The synthesis method is similar to Example 1, except that in the first step, m-chloroaniline is used instead of m-fluoroaniline, and the yield is 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com