Novel B-site five-membered high-entropy perovskite type oxide material and preparation method thereof
A perovskite-type and oxide technology, which is applied in chemical instruments and methods, nickel compounds, cobalt compounds, etc., can solve problems such as perovskite-type high-entropy oxides that have not yet been retrieved, and achieve easy regulation, convenient operation, The effect of high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
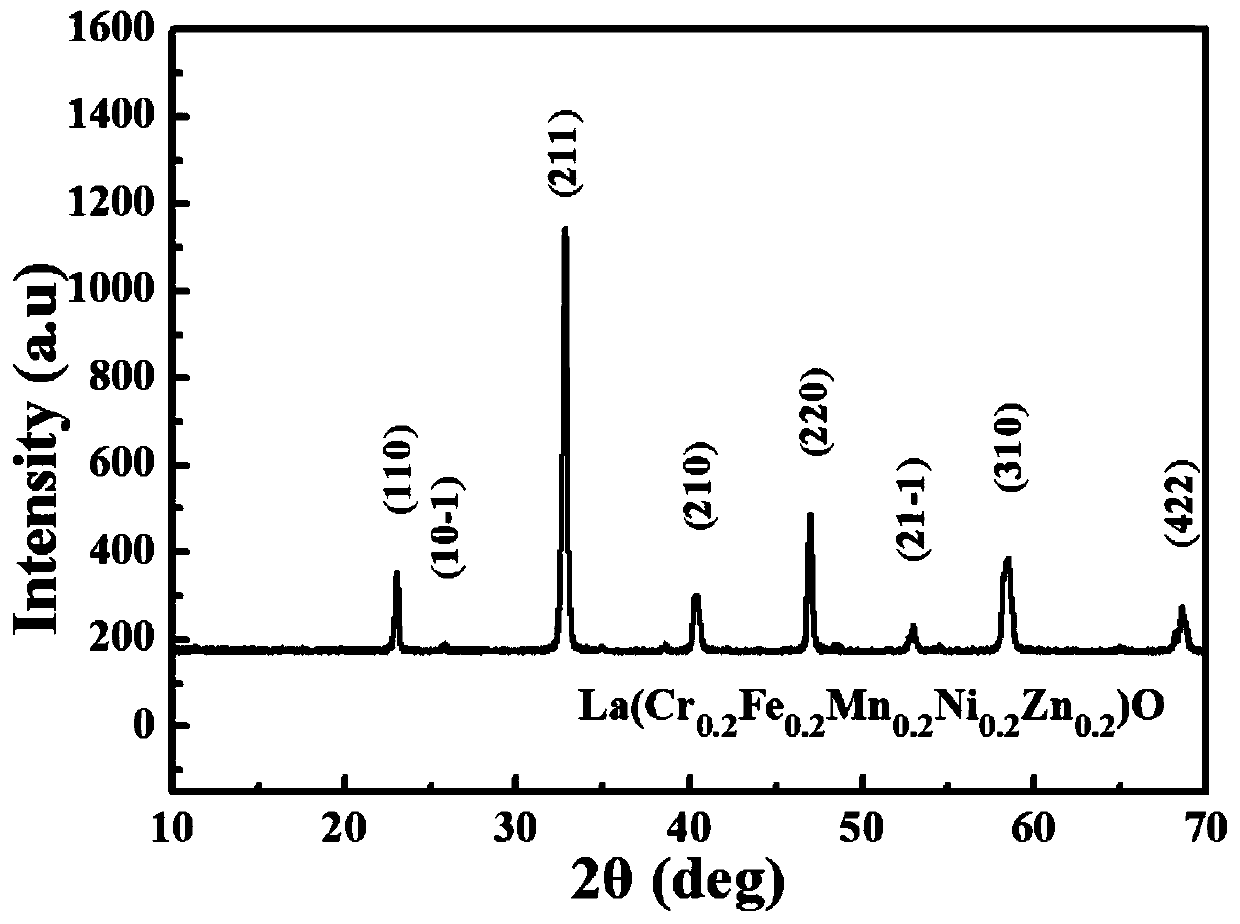
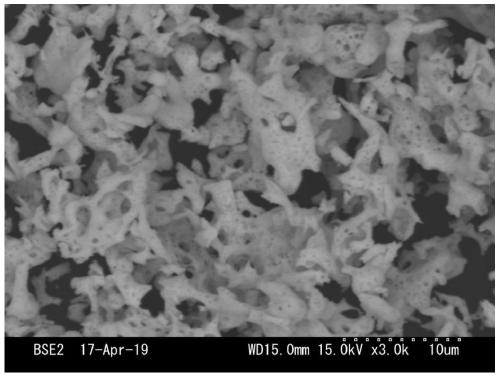
[0028] A new type of perovskite-type high-entropy oxide powder with B-site five-element high-entropy was prepared by solution combustion method. The chemical composition of the high-entropy oxide powder is La(Cr 0.2 Fe 0.2 mn 0.2 Ni 0.2 Zn 0.2 )O 3 : Take by weighing corresponding rare earth nitrate and metal nitrate according to the stoichiometric ratio of molecular formula, be specifically the La(NO of 4.330g 3 ) 3 .9H 2 O, 0.8004g of Cr(NO 3 ) 3 .9H 2 O, 0.808g of Fe(NO 3 ) 3 .9H 2 O, 0.574g of Mn(NO 3 ) 2 .4H 2 O, 0.582g of Ni(NO 3 ) 2 .6H 2 O and 0.595g of Zn(NO 3 ) 2 .6H 2 O, dissolved in 10mL distilled water, stirred evenly at room temperature to obtain a mixed solution containing one rare earth cation and five metal cations; then weighed 6.006g glycine and added to the mixed solution, stirred evenly; then put the above transparent sol Dry in an oven at 100°C to obtain a viscous gel after evaporating water; finally put the above gel in a muffle furn...
Embodiment 2
[0030] A new type of perovskite-type high-entropy oxide powder with B-site five-element high-entropy was prepared by solution combustion method. The chemical composition of the high-entropy oxide powder is Y(Cr 0.2 Fe 0.2 mn 0.2 Mg 0.2 Ni 0.2 )O 3 : Take by weighing corresponding rare earth nitrate and metal nitrate according to the stoichiometric ratio of molecular formula, specifically the Y(NO of 2.794g 3 ) 3 .nH 2 O, 0.8004g of Cr(NO 3 ) 3 .9H 2 O, 0.808g of Fe(NO 3 ) 3 .9H 2 O, 0.574g of Mn(NO 3 ) 2 .4H 2 O, 0.513gMg(NO 3 ) 2 .6H 2 O and 0.582g of Ni(NO 3 ) 2 .6H 2 O, dissolved in 10mL distilled water, stirred evenly at room temperature to obtain a mixed solution containing a rare earth cation and six metal cations; then weighed 6.004g of tartaric acid and added it to the mixed solution, stirred evenly; then put the above transparent sol Dry in an oven at 100°C to obtain a viscous gel after evaporating water; finally put the above gel in a gold furnac...
Embodiment 3
[0032] A new type of perovskite-type high-entropy oxide powder with B-site five-element high-entropy was prepared by solution combustion method. The chemical composition of the high-entropy oxide powder is Pr(Cr 0.2 Fe 0.2 mn 0.2 co 0.2 Zn 0.2 )O 3 : Take by weighing corresponding rare earth nitrate and metal nitrate according to the stoichiometric ratio of molecular formula, specifically 3.269g of Pr(NO 3 ) 3 .9H 2 O, 0.8004g of Cr(NO 3 ) 3 .9H 2 O, 0.808g of Fe(NO 3 ) 3 .9H 2 O, 0.574g of Mn(NO 3 ) 2 .4H 2 O, 0.582g of Co(NO 3 ) 2 .6H 2 O and 0.595g of Zn(NO 3 ) 2 .6H 2 O, dissolved in 8mL distilled water, stirred evenly at room temperature to obtain a mixed solution containing a rare earth cation and six metal cations; then weighed 1.921g of citric acid and added to the mixed solution, stirred evenly; then the above transparent sol Dry in an oven at 150°C to obtain a viscous gel after evaporating water; finally place the above gel in a muffle furnace an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com