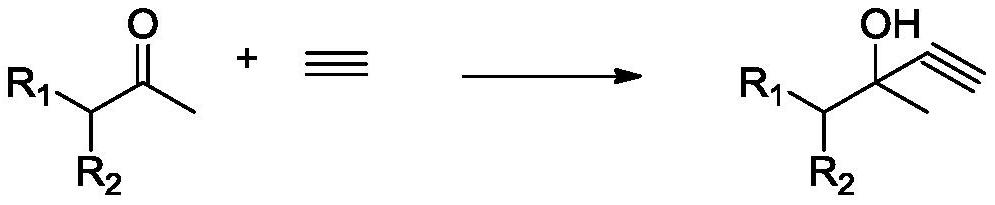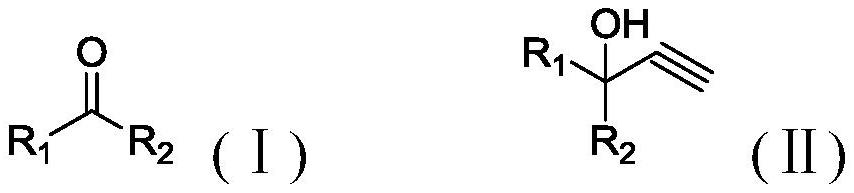A kind of acetylation method
A technology of ethynylation and acetylenic alcohol, applied in the field of ethynylation, can solve the problems of expensive catalyst, long reaction time, difficult to recycle, etc., and achieve the effect of moderate reaction rate, mild reaction, easy control, and promotion of dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
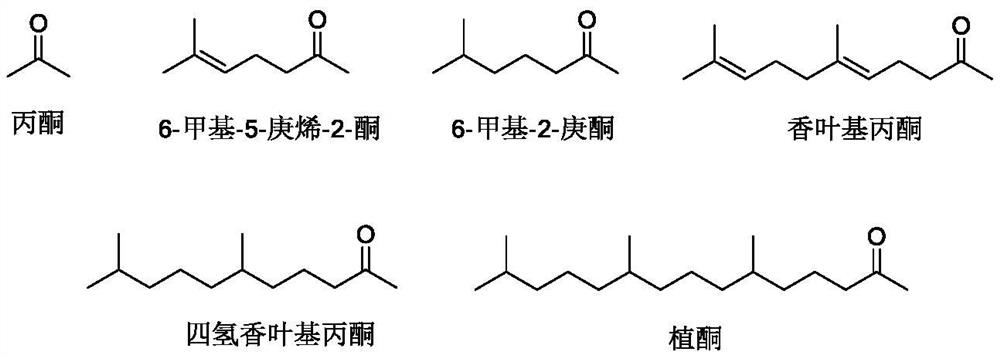
[0041] First, add N,N-dimethylformamide 126g, sodium methoxide 1.62g (0.03mol), sodium cyanide 0.49g (0.01mol) to the autoclave, seal the autoclave and replace the autoclave with nitrogen three times, and then feed The acetylene was replaced three times, and the acetylene pressure did not exceed 0.15MPa (gauge pressure). Turn on the stirring and set the temperature to 10°C, adjust the pressure of the pressure reducing valve of the acetylene cylinder to 0.1MPa (gauge pressure), and introduce acetylene gas. After the pressure of the reactor is stabilized at 0.1MPa (gauge pressure), use the feed pump to add 6-methyl-5-hepten-2-one 126g (1mol), the feeding time was controlled at 1h. The reaction temperature was controlled to 10°C, and the 6-methyl-5-hepten-2-one feeding was completed and then reacted for 2 hours, and then the pressure was released to release acetylene gas. 60 g of pure water was added to the reaction solution for extraction, the aqueous phase was retained, 30 g o...
Embodiment 2
[0045] First add N,N-dimethylformamide 189g, potassium ethoxide 3.40g (0.05mol), sodium cyanide 1.47g (0.03mol) to the autoclave, use nitrogen to replace the autoclave 3 times after sealing the autoclave, and then feed The acetylene was replaced three times, and the acetylene pressure did not exceed 0.15MPa (gauge pressure). Turn on the stirring and set the temperature to 15°C, adjust the pressure of the pressure reducing valve of the acetylene cylinder to 0.09MPa (gauge pressure), and introduce acetylene gas. After the pressure of the reactor is stabilized at 0.09MPa (gauge pressure), use the feed pump to add 6-methyl-5-hepten-2-one 126g (1mol), the feeding time was controlled at 1h. The reaction temperature was controlled to 15°C, and the 6-methyl-5-hepten-2-one feeding was completed, and the reaction was performed for 2 hours, and the pressure was released to release acetylene gas. 90 g of pure water was added to the reaction solution for extraction, the aqueous phase was ...
Embodiment 3
[0049] First, add N,N-dimethylformamide 189g, sodium ethoxide 0.68g (0.01mol), potassium cyanide 0.325g (0.005mol) to the autoclave, seal the autoclave and replace the autoclave with nitrogen three times, then feed The acetylene was replaced three times, and the acetylene pressure did not exceed 0.15MPa (gauge pressure). Turn on the stirring and set the temperature to 30°C, adjust the pressure of the pressure reducing valve of the acetylene cylinder to 0.08MPa (gauge pressure), and introduce acetylene gas. After the pressure of the reactor is stabilized at 0.08MPa (gauge pressure), use the feed pump to add 6-methyl-5-hepten-2-one 126g (1mol), the feeding time was controlled at 1h. The reaction temperature was controlled to 30°C, the 6-methyl-5-hepten-2-one feeding was completed, and the reaction was performed for 6 hours, and the pressure was released to release acetylene gas. 30 g of pure water was added to the reaction solution for extraction, the aqueous phase was retained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com