A method for simultaneously detecting the contents of seven components in Liwei capsules
A Liwei Capsule and Content Technology, which is applied to measurement devices, material separation, analysis of materials, etc., can solve the problems of large differences in physical and chemical properties of chemical components, inability to detect chemical component content, and complex prescription composition, and achieves high peak area, Accurate and fast effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The selection of embodiment 1 sample preparation method
[0023] Method 1: Take the contents of the capsule, mix well, take about 1.0g, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 50ml of methanol precisely, seal it tightly, weigh it, and ultrasonically treat it (power 250W, frequency 40kHz) for 30 Minutes, let cool, then weigh again, make up the lost weight with methanol, shake well, filter, accurately take 10ml of the filtrate in a conical flask, add 5ml of 2.5mol / l sodium hydroxide, and heat in a water bath at 80°C After 30 minutes, let it cool, add 2.5mol / l hydrochloric acid to adjust the pH value to 2~3, shake and extract twice with ethyl acetate, 10ml each time, combine the ethyl acetate solution, recover the solvent under reduced pressure to dryness, and transfer the residue to In a 10ml measuring bottle, add methanol to the mark, shake well, filter, and take the filtrate to obtain the final product.
[0024] Method 2: Take the contents of t...
Embodiment 2
[0031] The selection of embodiment 2 wavelength
[0032] Sample solution preparation method: the same as method 1 in Example 1.
[0033] Detection wavelength: 210nm, 230nm, 254nm
[0034] Other conditions are identical with embodiment 1.
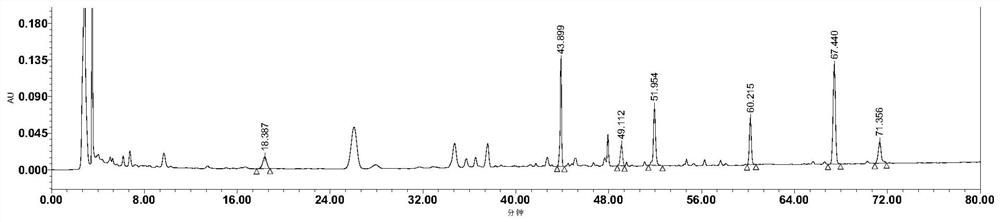
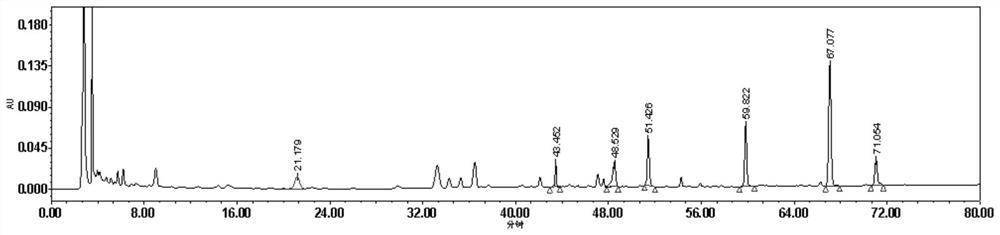
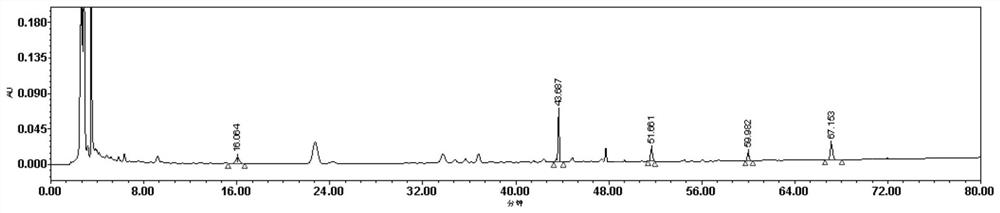
[0035] result: image 3 is the chromatogram of the detection wavelength of 210nm, and figure 1 In comparison, not only are few components detected, but the area of each peak is less than figure 1 . Figure 4 It is the chromatogram of the detection wavelength of 254nm. It can be seen from the figure that there is no notoginseng saponin R in about 18 minutes 1 Chromatographic peak, ginsenoside Rg around 44 minutes 1 Chromatographic peak areas are much lower than figure 1 Ginsenoside Rg 1 of the peak area. Therefore 230nm is the optimum detection wavelength of the present invention.
Embodiment 3
[0036] Embodiment 3 mobile phase selection
[0037] Sample solution preparation method: the same as method 1 in Example 1.
[0038] Mobile phase 1: 5% tetrahydrofuran acetonitrile solution as mobile phase A, 0.1% phosphoric acid aqueous solution as mobile phase B
[0039] Mobile phase 2: Acetonitrile solution is mobile phase A, water is mobile phase B
[0040] Mobile phase 3: methanol solution is mobile phase A, 0.1% phosphoric acid solution is mobile phase B
[0041] Other conditions are identical with embodiment 1.
[0042] result: Figure 5It is the chromatogram after gradient elution of mobile phase 2, as can be seen from the figure, the mobile phase peak resolution and peak shape without tetrahydrofuran and phosphoric acid are obviously poor, wherein ginsenoside Rg 1 The chromatographic peaks and adjacent peaks are not completely separated, and aloe-emodin is not completely separated from adjacent peaks. Figure 6 It is the chromatogram after adopting mobile phase 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com