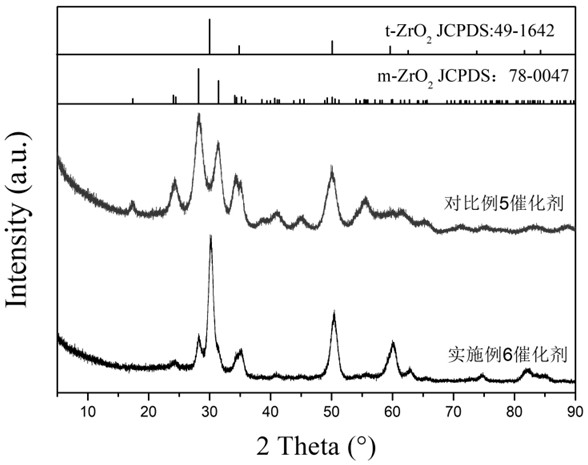
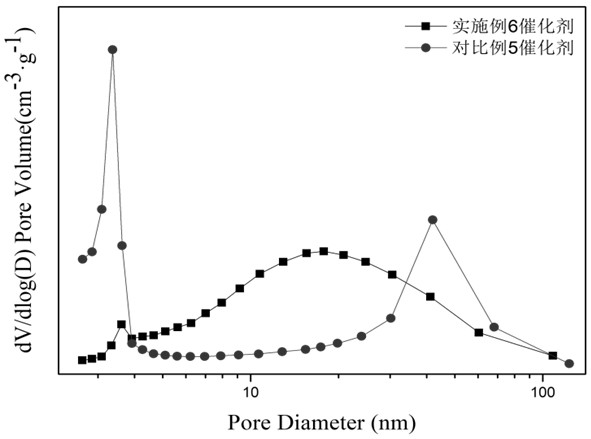
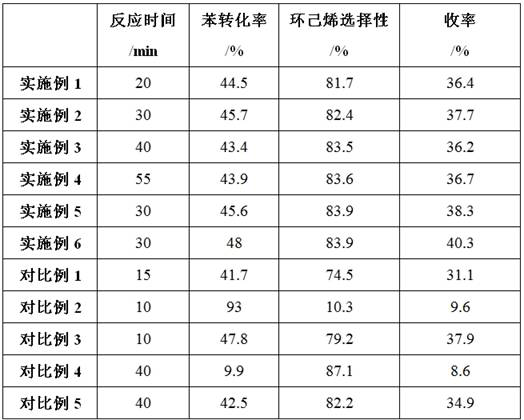
A supported Ru-based catalyst supported by molybdenum oxide-zinc oxide-zirconia composite oxide
A composite oxide, carrier-supported technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, catalyst activation/preparation, etc. The products are difficult to separate, and the amount of precious metal Ru is large, so as to achieve uniform loading and activation, improved selectivity and stability, and improved selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation of zirconia
[0023] To 125 g 750 mL of ZrOCl 2 ·8H 2 Gradually add 12.5 wt% ammonia water to O aqueous solution until the pH of the solution reaches 10, and stir and reflux at 100 °C for 48 h to obtain a white precipitate, which is washed three times with deionized water and twice with absolute ethanol, and dried at 60 °C 24 h, and then calcined at 800 °C for 5 h to obtain zirconia powder. ZrO 2 Characterization shows: ZrO 2 The specific surface area is 83.7 m 2 / g, the pore volume is 0.38 cm 3 / g -1 , with an average pore diameter of 18.3 nm.
[0024] (2) Preparation of catalyst
[0025] Weigh 4.5 g of the above ZrO 2 Dispersed in 0.029 g (NH 4 ) 6 Mo 7 o 24 ∙4H 2 O and 0.174 g of Zn ( NO 3 ) 2 ∙6H 2O into 10 mL aqueous solution, stirred for 0.5 h, added 10 wt% ammonium bicarbonate solution to adjust the pH to 8, continued to stir for 1 h, and then dried in a drying oven at 100 °C for 2 h, and then dried in a muffle furnace at 300 °C ...
Embodiment 2
[0027] (1) Zirconia same as Example 1
[0028] (1) Preparation of catalyst
[0029] Weigh 4.5 g ZrO 2 Dissolved in 0.029 g (NH 4 ) 6 Mo 7 o 24 ∙4H 2 O and 0.348 g Zn( NO 3 ) 2 ∙6H 2 O into 10 mL aqueous solution, stirred for 0.5 h, added 10 wt% ammonium bicarbonate solution to adjust the pH to 8, continued to stir for 1 h, dried in a drying oven at 100 °C for 2 h, and baked in a muffle furnace at 300 °C 1 h. Weigh 4 g of the above-mentioned carrier and dissolve it in 50 mL of deionized water. Under stirring conditions, dissolve 0.602 g of 20 mL of RuCl 3 ·3H 2 O aqueous solution and 5 wt% NaOH solution were added together to adjust the pH to 9.5, stirred for 3.5 h, and 0.315 g of 10 mL of NaBH was slowly added 4 Aqueous solution, stirred for 1h. The obtained precipitate was centrifuged and washed with deionized water until neutral; the precipitate was vacuum-dried at 80 °C for 24 h.
Embodiment 3
[0031] (1) Zirconia same as Example 1
[0032] (2) Preparation of catalyst
[0033] Weigh 4.5 g ZrO 2 Dissolve in 0.015 g (NH 4 ) 6 Mo 7 o 24 ∙4H 2 O and 0.523 g Zn( NO 3 ) 2 ∙6H 2 In the 10 mL aqueous solution prepared by O, stir for 0.5 h, add 10wt% ammonium bicarbonate solution to adjust the pH to 8, continue stirring for 1 h, then dry in a drying oven at 100 °C for 2 h, and bake in a muffle furnace at 300 °C 1 h. Weigh 4 g of the above-mentioned carrier and dissolve it in 50 mL of deionized water. Under stirring conditions, dissolve 0.602 g of 20 mL of RuCl 3 ·3H 2 O aqueous solution and 5 wt% NaOH solution were added together to adjust the pH to 9.5, stirred for 3.5 h, and 0.315 g of 10 mL of NaBH was slowly added 4 aqueous solution, stirred for 1 h. The obtained precipitate was centrifuged and washed with deionized water until neutral; the precipitate was vacuum-dried at 80 °C for 24 h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com