Method for synthesizing cyclohexylbenzene by using fixed bed
A technology of cyclohexylbenzene and fixed bed, which is applied in the chemical industry and can solve the problem of low selectivity of cyclohexylbenzene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of hydroalkylation catalyst
[0034] At room temperature, weigh a certain amount of RuCl 3 ·3H 2 Dissolve O in deionized water, add molecular sieves, stir for 10 minutes, heat up to 60°C, add 10% ammonium carbonate solution dropwise, stop dropping when pH>8, stir for 30 minutes, measure pH value, if pH 8, then stirred for 30 min, and measured the pH value, and so on until pH > 8, and then continued to stir for 2 h. Filtration, washing, drying, roasting, tablet forming, sieving, and standby.
[0035] (2) Preparation of transalkylation catalyst
[0036] Weigh 30g of USY molecular sieve with a silicon-aluminum ratio of 12, add 120g of citric acid aqueous solution with a mass concentration of 2%, treat at 80°C for 4h, filter, wash, dry, roast at 580°C for 4h, press into tablets, sieve, and set aside.
[0037] (3) Reaction process
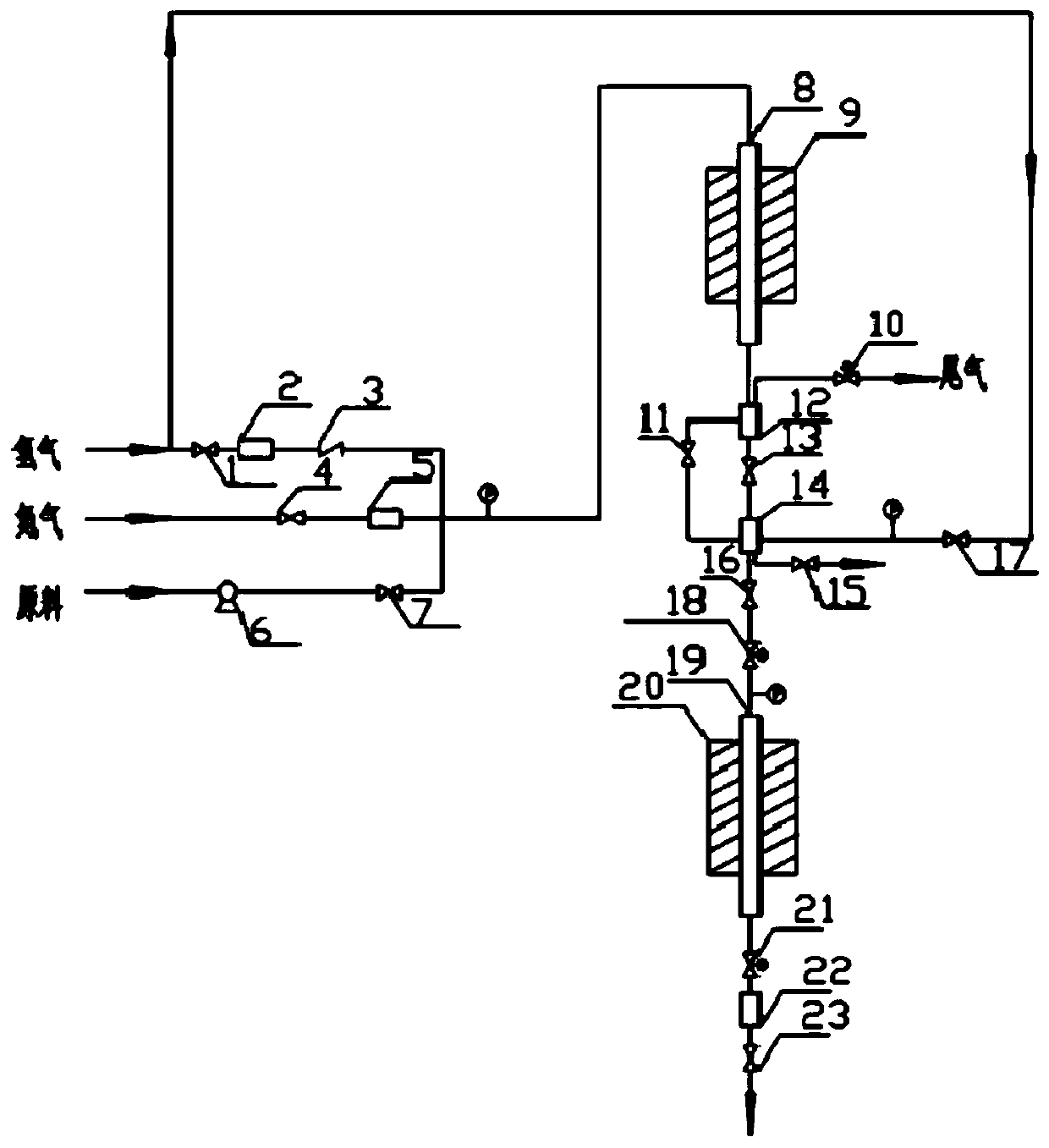
[0038] Firstly, the hydroalkylation catalyst and the transalkylation catalyst were loaded respectively, and the two ends of ...
Embodiment 2
[0044] Catalyst preparation is the same as in Example 1.
[0045] reaction process
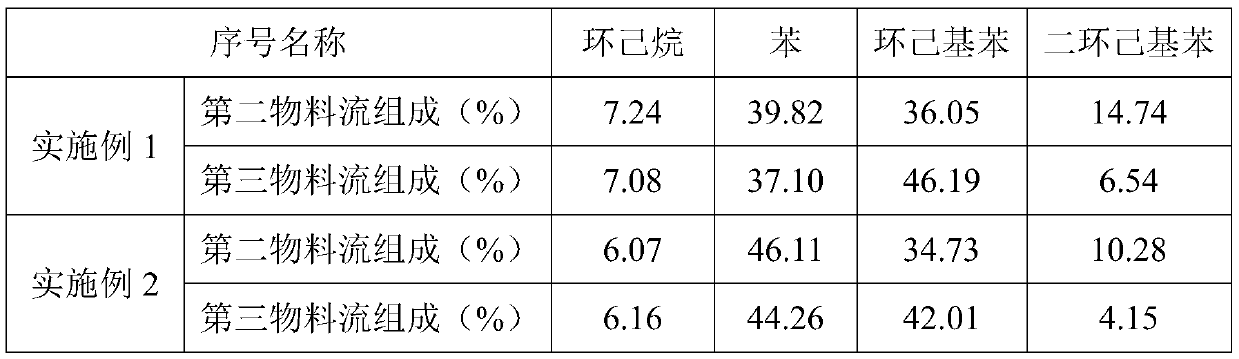
[0046] Firstly, the hydroalkylation catalyst and the transalkylation catalyst were loaded respectively, and the two ends of the hydroalkylation reaction tube and the transalkylation reaction tube were respectively filled with quartz sand, and the loading amount of the catalyst was 4 g. The hydroalkylation catalyst is reduced, and the reduction temperature is 250°C. Hydroalkylation reaction conditions: benzene space velocity is 2.0h -1 , the reaction temperature is 180°C, the reaction pressure is 2.0MPa, and the molar ratio of hydrogen to benzene is 0.6. Transalkylation reaction conditions: reaction temperature 240°C, reaction pressure 2.0MPa. After 24 hours of reaction, samples were taken and analyzed. The mass percentages of cyclohexane, benzene, cyclohexylbenzene and dicyclohexylbenzene in the second stream and the third stream are shown in Table 1.
[0047] Table 1
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com