Tin-doped lithium-rich manganese-based positive electrode material and preparation method thereof
A lithium-rich manganese-based, positive electrode material technology, applied in the direction of battery electrodes, structural parts, electrical components, etc., can solve problems that have not been reported in the literature, achieve good controllability and reproducibility, simple operation, and improved stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
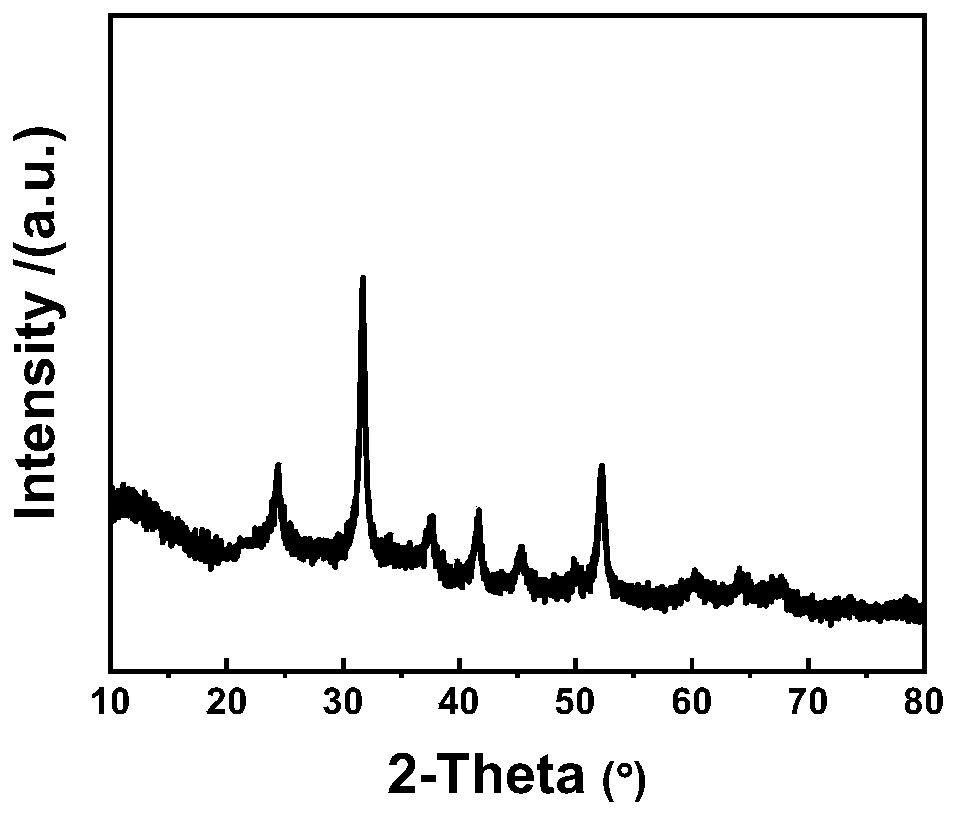
Image
Examples
Embodiment 1
[0027] Co-precipitation synthesis of Li-rich material Li[Li 0.2 mn 0.53 Ni 0.13 co 0.13 sn 0.01 ]O 2 .
[0028] Weigh 4.659g NiCl 2 ·6H 2 O, 15.827g MnCl 2 4H 2 O, 4.663g CoCl 2 ·6H 2 O, 0.338gSnCl 2 2H 2 O was dissolved in 100ml of deionized water and stirred evenly to make a mixed salt solution; then weighed 13.040g of Na2 CO 3 Dissolve in 100ml of deionized water, and add 2.0ml of ammonia water with a concentration of 25% therein and mix well to prepare a mixed alkali solution. Add 50ml of deionized water into the reactor as the bottom liquid, and then use the peristaltic pump to add the above-mentioned mixed alkali solution and the above-mentioned mixed salt solution to the reactor at the same time, and adjust the reaction in the whole process by controlling the amount of mixed alkali solution added. The pH of the solution was 8.0, and the temperature of the reactor was controlled at 55° C. while stirring continuously at a stirring speed of 800 rpm.
[0029]...
Embodiment 2
[0034] Co-precipitation method to synthesize Li-rich material Li[Li 0.2 mn 0.52 Ni 0.13 co 0.13 sn 0.02 ]O 2 .
[0035] Weigh 4.754g NiCl 2 ·6H 2 O, 15.827g MnCl 2 4H 2 O, 4.759g CoCl 2 ·6H 2 O, 0.694gSnCl 2 2H 2 O was dissolved in 100ml of deionized water and stirred evenly to make a mixed salt solution; then weighed 13.040g of Na 2 CO 3 Dissolve in 100ml of deionized water, and add 2.0ml of ammonia water with a concentration of 25% therein and mix well to prepare a mixed alkali solution. Add 50ml of deionized water into the reactor as the bottom liquid, and then use the peristaltic pump to add the above-mentioned mixed alkali solution and the above-mentioned mixed salt solution to the reactor at the same time, and adjust the reaction in the whole process by controlling the amount of mixed alkali solution added. The pH of the solution was 8.0, and the temperature of the reactor was controlled at 55° C. while stirring continuously at a stirring speed of 800 rpm....
Embodiment 3
[0042] Weigh 5.257g NiSO 4 ·6H 2 O, 13.522g MnSO 4 ·H 2 O, 5.622g CoSO 4 ·7H 2 O, 0.694gSnCl 2 2H 2 O was dissolved in 100ml of deionized water and stirred evenly to make a mixed salt solution; then weighed 13.040g of Na 2 CO 3 Dissolve in 100ml of deionized water, and add 2.0ml of ammonia water with a concentration of 25% therein and mix well to prepare a mixed alkali solution. Add 50ml of deionized water into the reactor as the bottom liquid, and then use the peristaltic pump to add the above-mentioned mixed alkali solution and the above-mentioned mixed salt solution to the reactor at the same time, and adjust the reaction in the whole process by controlling the amount of mixed alkali solution added. The pH of the solution was 7.8, and the temperature of the reactor was controlled at 60° C. while stirring continuously at a stirring speed of 900 rpm.
[0043] After the above two mixed solutions stopped adding, continue to stir for 6 hours, then stand and age for 20 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com