Alpha-ketothioamide compound and synthesis method thereof
A technology for ketone thioamides and amine compounds, applied in the field of α-ketothioamide compounds and their synthesis, can solve the problems of harsh conditions, generation of phosphorus waste, etc., and achieves low preparation cost, short reaction time, and substrates. wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
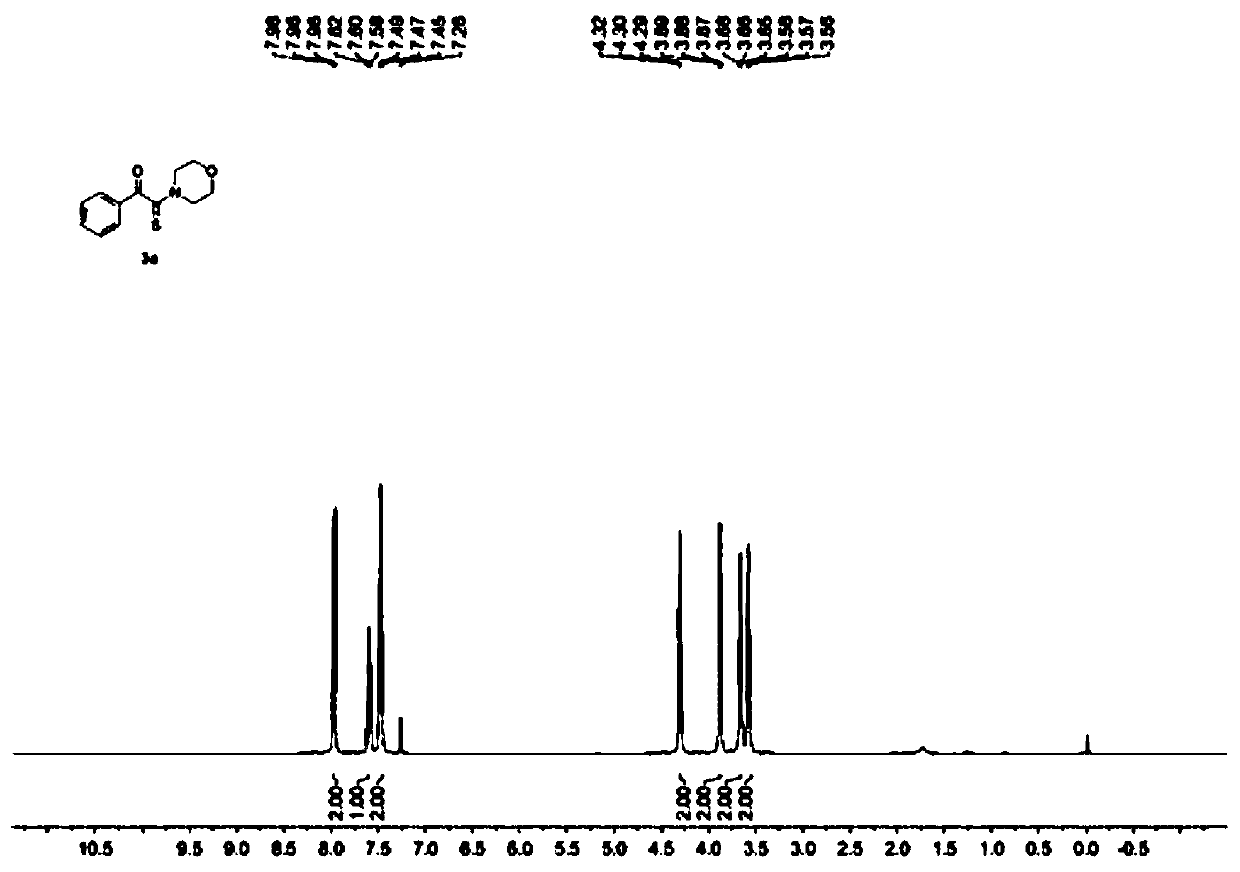
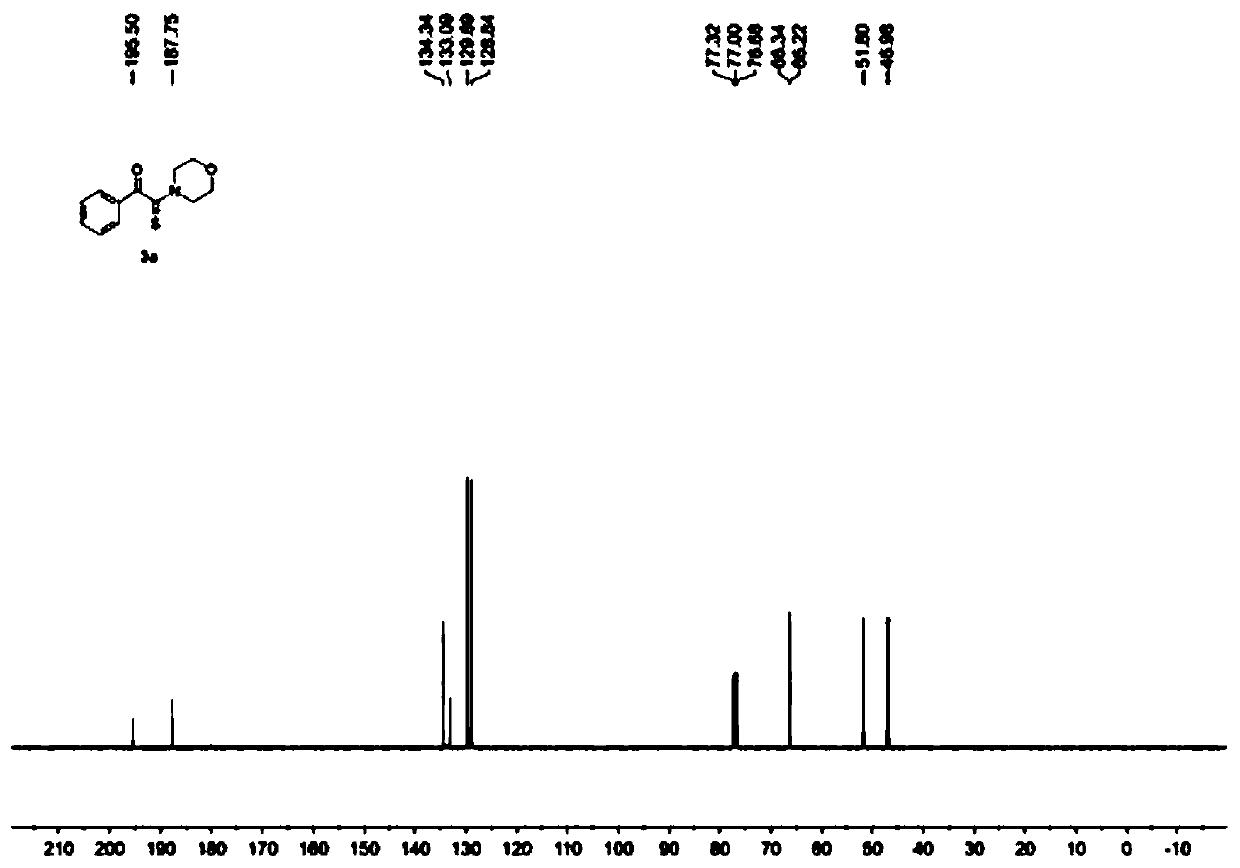
[0038] Add 1mmol of α-azidoacetophenone, 3mmol of morpholine, 2mmol of elemental sulfur and 5ml of solvent THF into a 50mL round-bottomed flask, and react with magnetic stirring at room temperature for 3 hours. and saturated brine, left to stand to obtain an organic layer and an aqueous layer containing the product, after liquid separation, the organic layer was dried with anhydrous sodium sulfate, and the extraction solvent was evaporated under reduced pressure to obtain a crude product, which was washed with ethyl acetate / petroleum Ether = 1:15 (v / v) was used as the eluent for column separation and purification, and the eluent was evaporated under reduced pressure to obtain the yellow solid product 2-morpholino-1-phenyl-2-thioxoethanone with a yield of 94%. Such as figure 1 and figure 2 Shown, respectively, the obtained product 1 H NMR characterization spectrum and 13 C NMR characterization spectrum, the chemical shifts of the peaks corresponding to the product 2-morphol...
Embodiment 2
[0040] Add 1mmol of α-azidoacetophenone, 1.2mmol of morpholine, 1.5mmol of elemental sulfur and 5ml of solvent DMF into a 50mL round-bottomed flask, and react with magnetic stirring at room temperature for 0.5 hours. Ethyl ester and saturated brine were mixed, and the organic layer and water layer containing the product were obtained by standing still. After liquid separation, the organic layer was dried with anhydrous sodium sulfate, and the extraction solvent was evaporated under reduced pressure to obtain a crude product. The crude product was washed with ethyl acetate / Petroleum ether=1:20 (v / v) was used as the eluent for column separation and purification, and the eluent was evaporated under reduced pressure to obtain a yellow solid product 2-morpholino-1-phenyl-2-thioxoethanone with a yield of 86%.
Embodiment 3
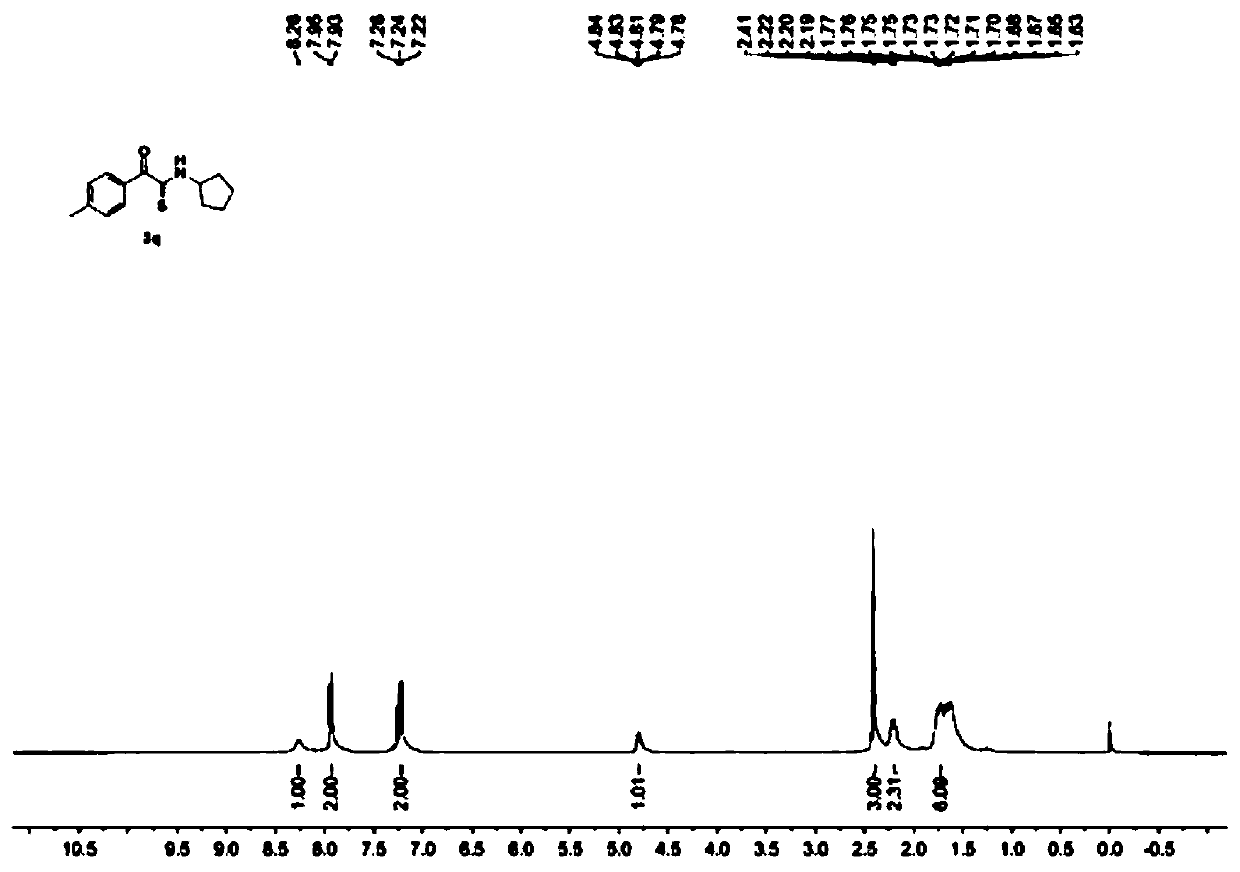
[0042] Add 1mmol of α-azidoacetophenone and 5mmol of cyclopentylamine to a 50mL round bottom flask, add 4mmol of elemental sulfur and 5ml of solvent DMSO, and react with magnetic stirring at room temperature for 3 hours. Esters and saturated brine were mixed, and the organic layer and water layer containing the product were obtained by standing still. After liquid separation, the organic layer was dried with anhydrous magnesium sulfate, and the extraction solvent was evaporated under reduced pressure to obtain a crude product. The crude product was washed with ethyl acetate / Petroleum ether = 1:10 (v / v) was used as eluent for column separation and purification, and the eluent was evaporated under reduced pressure to obtain a yellow solid product N-cyclopentyl-2-oxo-2-(p-tolyl)sulfur On behalf of acetamide, the yield was 88%. Such as figure 1 and figure 2 Shown, respectively, the obtained product 1 H NMR characterization spectrum and 13 C NMR characterization collection, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com