Aluminum distearate coating method for improving stability of perovskite quantum dots
A technology of aluminum distearate and quantum dots, which is applied in the field of optoelectronic material preparation, can solve the problems of unfavorable applications, poor thermal stability of size polymers, etc., achieve passivation of surface defects, excellent optical and colloidal stability, and reduce erosion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
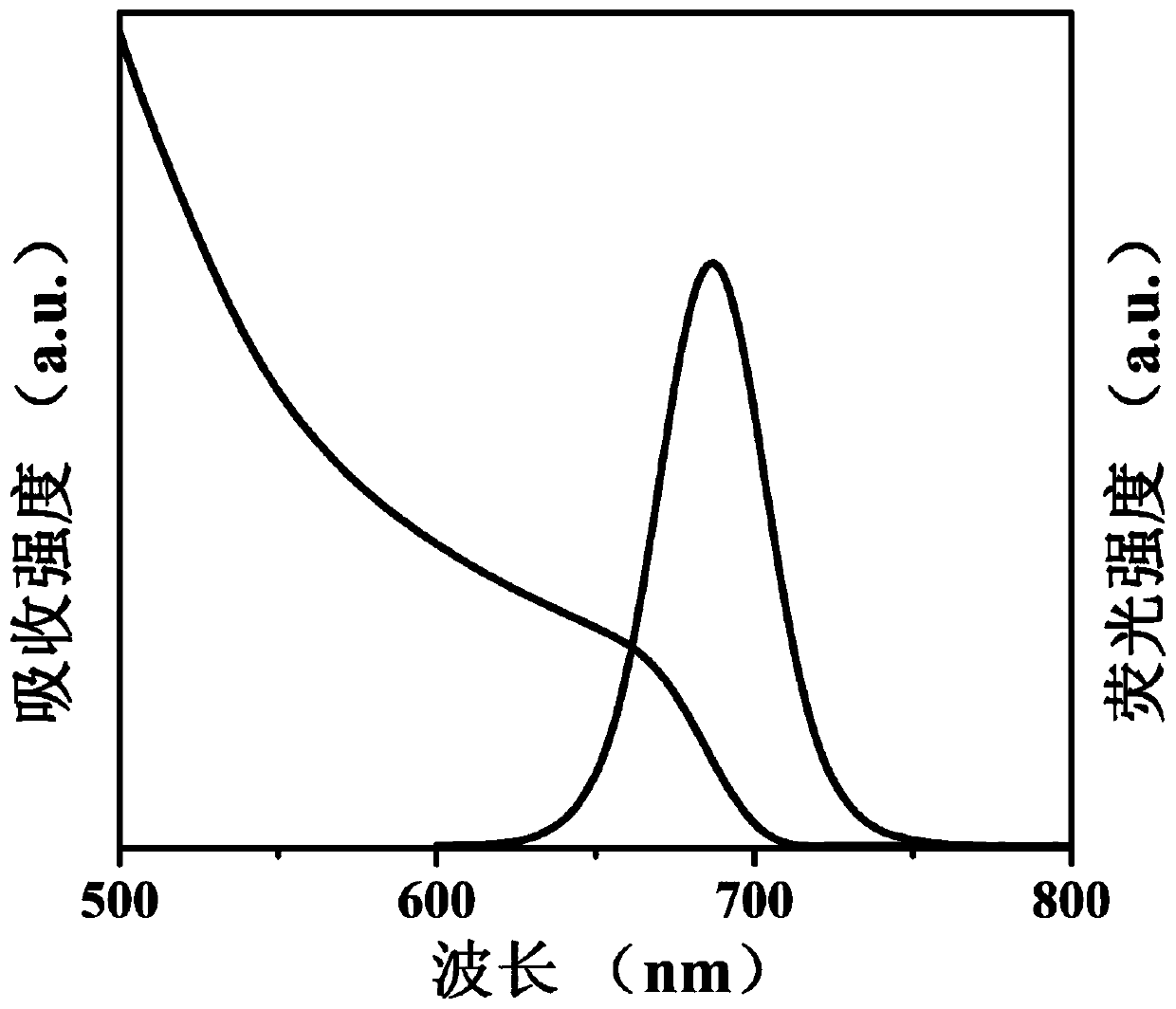
[0054] (a) 0.1628g Cs 2 CO 3 , 0.5mL oleic acid and 8mL 1-octadecene were added to a 50mL three-necked flask, the reaction system was heated to 120°C under vacuum and kept for 1 hour, then nitrogen was introduced into the reaction system and heated to 150°C until Cs 2 CO 3 Completely dissolved to obtain a clear and transparent cesium source solution.
[0055] (b) 0.0867g PbI 2 , 5mL 1-octadecene, 0.7mL oleic acid, 0.7mL oleylamine and 55mg aluminum distearate were added to a 50mL three-necked flask, and the reaction system was heated to 120°C under vacuum until the reactants were completely dissolved and kept for 1 hour , then feed nitrogen into the reaction system and heat to 160 ° C, and quickly inject 0.4 ml of cesium source prepared in (a) into the reactant solution, and after 5 seconds of reaction, cool the solution to room temperature with an ice-water bath to obtain the crude product CaPbI 3 @Al-0.5 quantum dot dispersion.
[0056] (c) the crude product CsPbI 3 @...
Embodiment 2
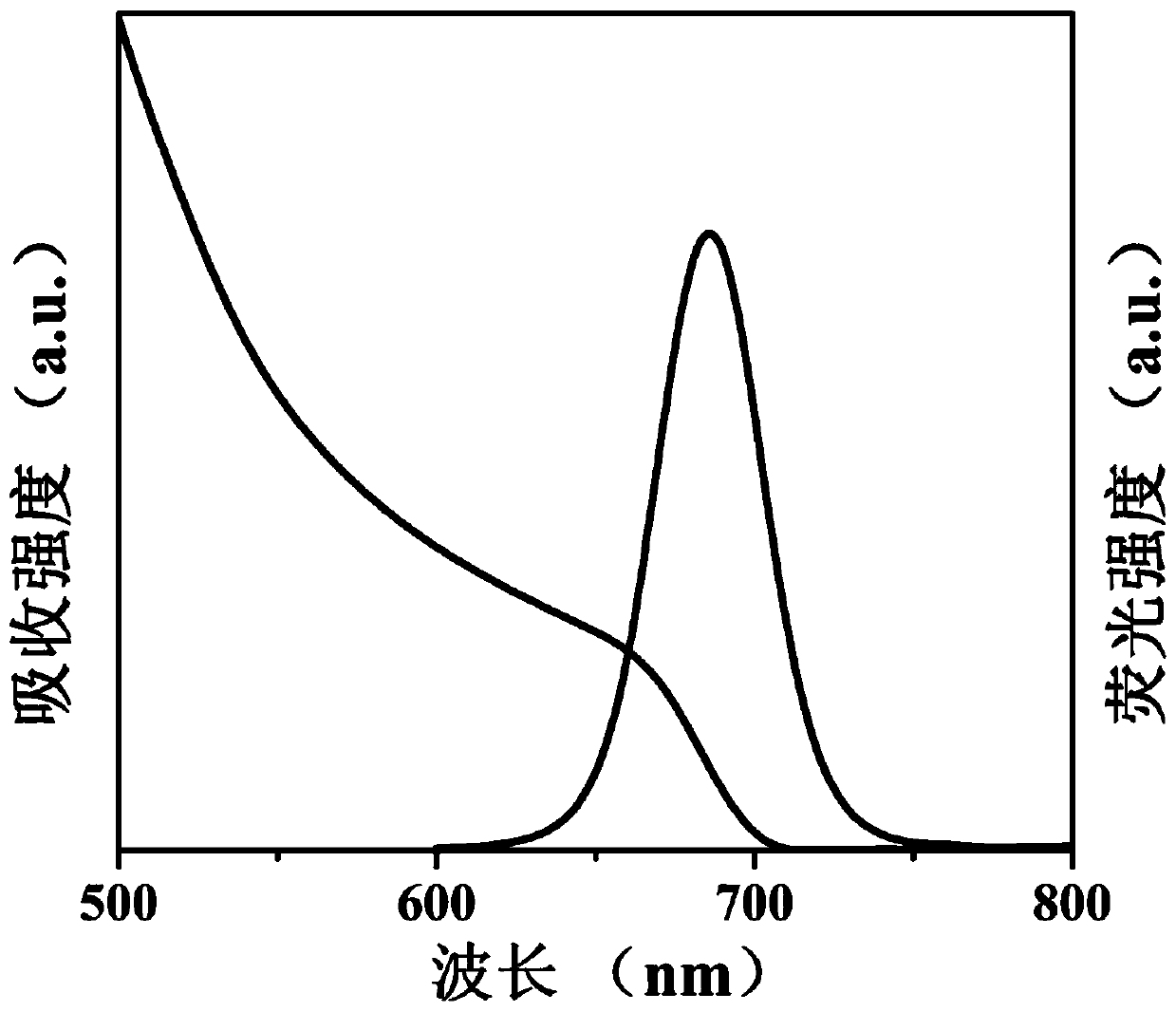
[0059] (a) 0.1628g Cs 2 CO 3 , 0.5mL oleic acid and 8mL 1-octadecene were added to a 50mL three-necked flask, the reaction system was heated to 120°C under vacuum and kept for 1 hour, then nitrogen was introduced into the reaction system and heated to 150°C until Cs 2 CO 3 Completely dissolved to obtain a clear and transparent cesium source solution.
[0060] (b) 0.0867g PbI 2 , 5mL 1-octadecene, 0.7mL oleic acid, 0.7mL oleylamine and 110mg aluminum distearate were added to a 50mL three-necked flask, and the reaction system was heated to 120°C under vacuum until the reactants were completely dissolved and kept for 1 hour , then feed nitrogen into the reaction system and heat to 160 ° C, and quickly inject 0.4 ml of cesium source prepared in (a) into the reactant solution, and after 5 seconds of reaction, cool the solution to room temperature with an ice-water bath to obtain the crude product CaPbI 3 @Al-1 quantum dot dispersion.
[0061] (c) the crude product CsPbI 3 @A...
Embodiment 3
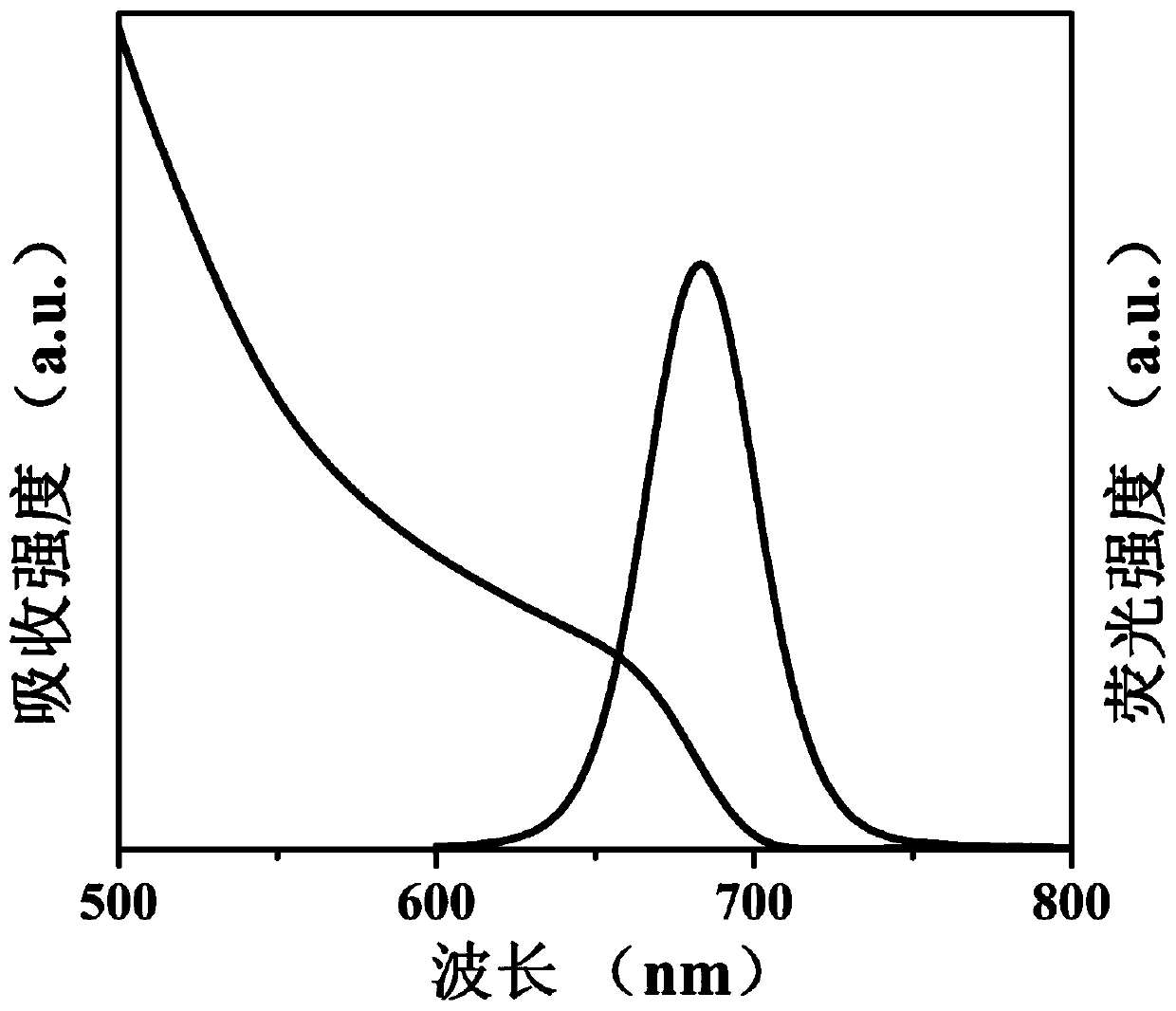
[0064] (a) 0.1628g Cs 2 CO 3 , 0.5mL oleic acid and 8mL 1-octadecene were added to a 50mL three-necked flask, the reaction system was heated to 120°C under vacuum and kept for 1 hour, then nitrogen was introduced into the reaction system and heated to 150°C until Cs 2 CO 3 Completely dissolved to obtain a clear and transparent cesium source solution.
[0065] (b) 0.0867g PbI 2 , 5mL 1-octadecene, 0.7mL oleic acid, 0.7mL oleylamine and 165mg aluminum distearate were added to a 50mL three-necked flask, and the reaction system was heated to 120°C under vacuum until the reactants were completely dissolved and kept for 1 hour , then feed nitrogen into the reaction system and heat to 160 ° C, and quickly inject 0.4 ml of cesium source prepared in (a) into the reactant solution, and after 5 seconds of reaction, cool the solution to room temperature with an ice-water bath to obtain the crude product CaPbI 3 @Al-1.5 quantum dot dispersion.
[0066] (c) the crude product CsPbI 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com