Novel esomeprazole strontium compound, and pharmaceutical composition and application thereof
A technology of esomeprazole strontium and meprazole strontium is applied in the field of medicine and achieves the effects of being convenient for storage and transportation, reducing manufacturing costs and costs, and being easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
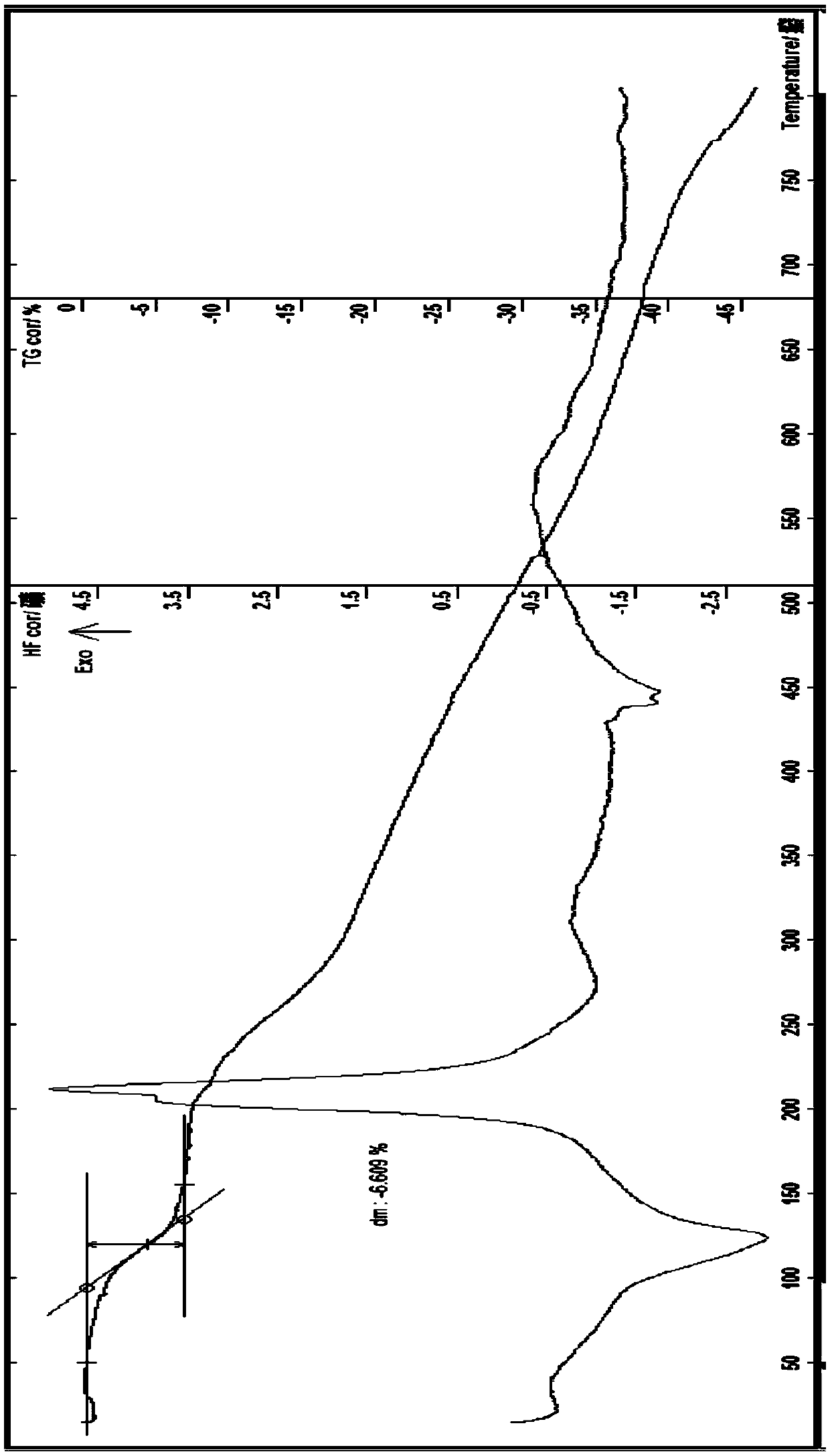
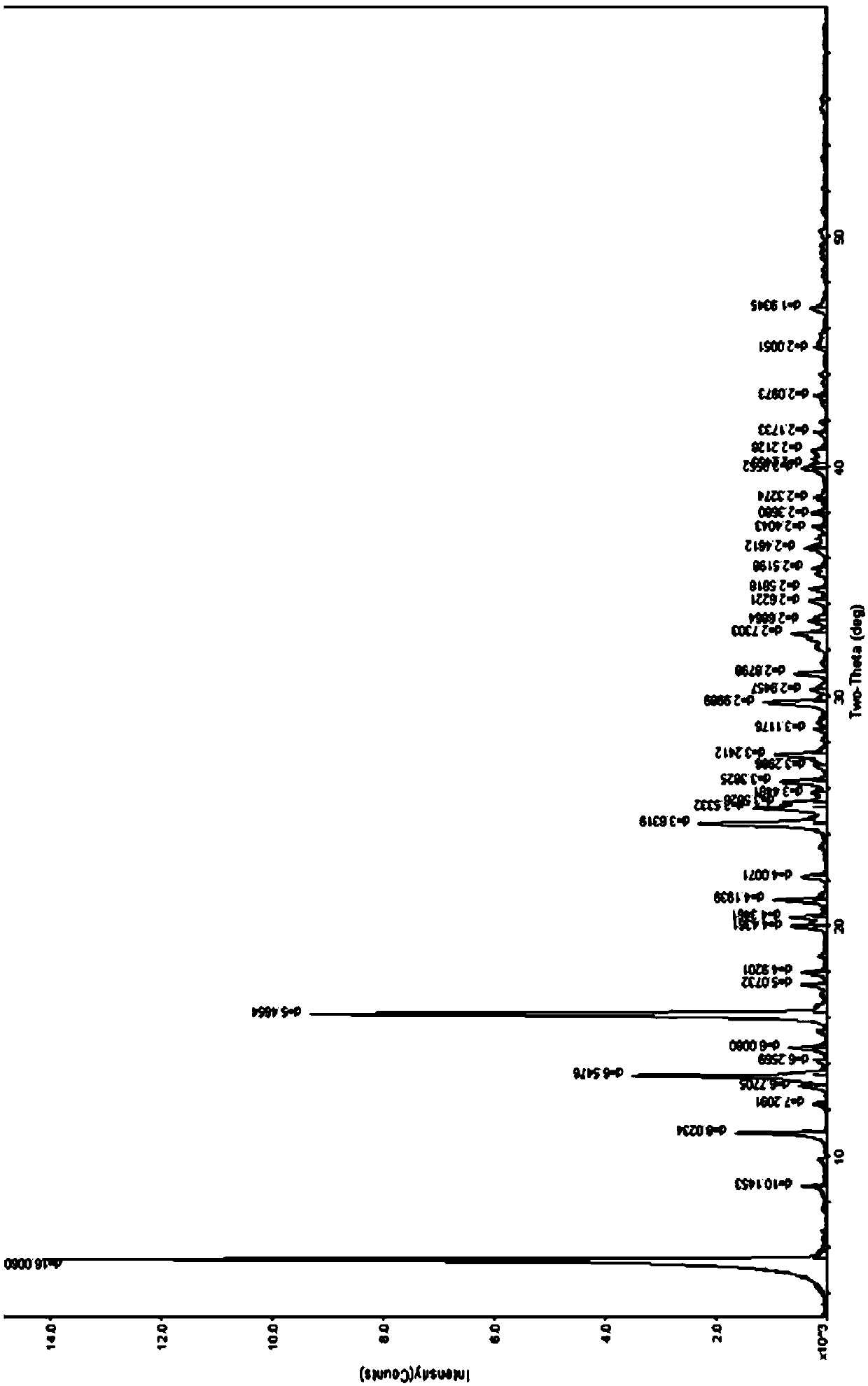
[0116] The preparation of embodiment 1 esomeprazole strontium 3 hydrate (ν type)
[0117] At room temperature, add 6.824g of esomeprazole sodium and 40ml of methanol to a 250ml flask, stir to dissolve, heat to about 40°C, then dropwise add about 16ml of methanol solution of 2.37g of strontium chloride hexahydrate, stir, about 10 A large amount of precipitates precipitated in about 12 minutes, continued to stir for about 12 minutes, took out the suction filter, washed a small amount of water and ethanol to remove the residual sodium chloride in the solid, and filtered the solid. Air-drying about 4h, obtain off-white solid 6.3g; HPLC: measure with two kinds of methods of content and related substance respectively, the main peak retention time of the HPLC of the product of this embodiment and esomeprazole or esomeprazole sodium reference substance main peak The HPLC retention time is consistent; Karl Fischer's method measures moisture to be 6.65%, thermal analysis: platform weigh...
Embodiment 2
[0118] The preparation of embodiment 2 esomeprazole strontium 3 hydrates (ν type)
[0119] At room temperature, add 6.91g of esomeprazole sodium and 40ml of methanol to a 250ml flask, stir to dissolve, add about 20ml of methanol solution of 2.38g of strontium chloride hexahydrate at about 40°C, stir to dissolve, stir, about After a large amount of white precipitate precipitated in about 10 minutes, continue to stir for 20 minutes, add about 2ml of water, continue to stir for 8 minutes, filter the object in the reaction bottle with suction, wash with a small amount of water and ethanol five times, and filter with suction, the obtained solid is diluted at about 46°C. Air-drying 5h, obtain off-white solid 6.2g; HPLC: measure with two kinds of methods of content and related substance respectively, the main peak retention time of its HPLC is consistent with the HPLC retention time of main peak of esomeprazole sodium reference substance; Moisture is 6.77%, thermal analysis: platform...
Embodiment 3
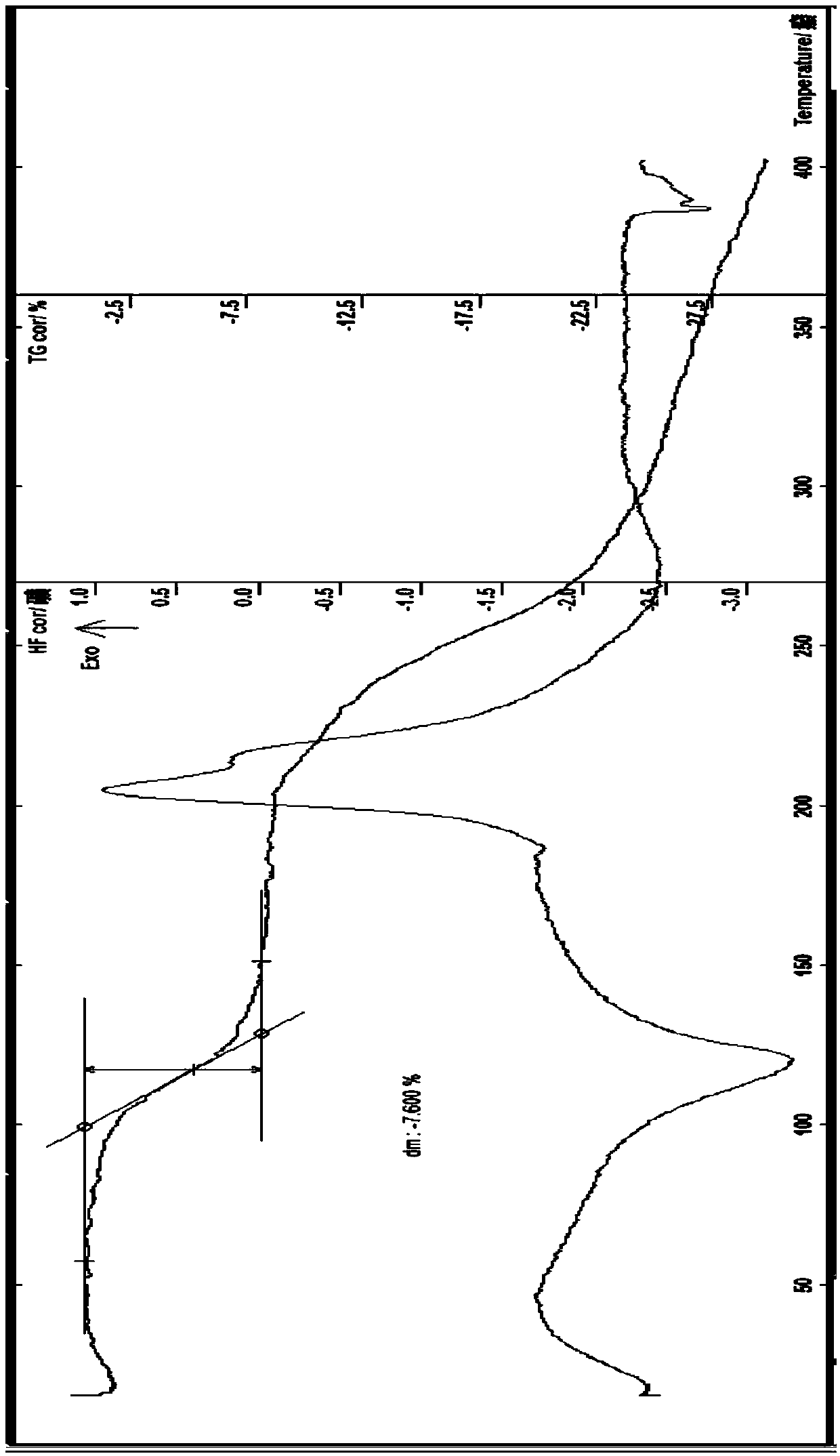
[0120] The preparation of embodiment 3 esomeprazole strontium 3.5 hydrate (ω type)
[0121] In a 500ml flask, add 13.8g of esomeprazole sodium (purity 99.9%), add 85ml of methanol, stir at about 40°C to dissolve, add 4.75g of strontium chloride hexahydrate, about 40ml of methanol solution, stir to dissolve, stir , A large amount of white precipitate precipitated in about 10 minutes, continue to stir for about 20 minutes, add about 10ml of water, continue to stir for about 10 minutes, suction filter the object in the reaction bottle, wash the solid with a small amount of water and ethanol to remove the residual sodium chloride in the solid, Suction filtration, the resulting solid was diluted and dried in a blast drying oven at about 30°C for 1h, and then dried at 45°C for about 2h to obtain 12.4g of off-white solid; HPLC: determined by two methods of content and related substances, the main peak of HPLC remained Time is consistent with the main peak retention time of esomeprazo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com