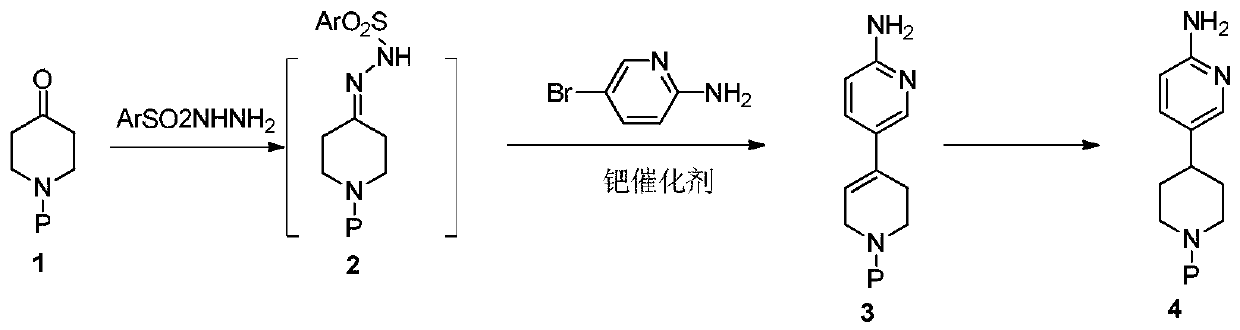
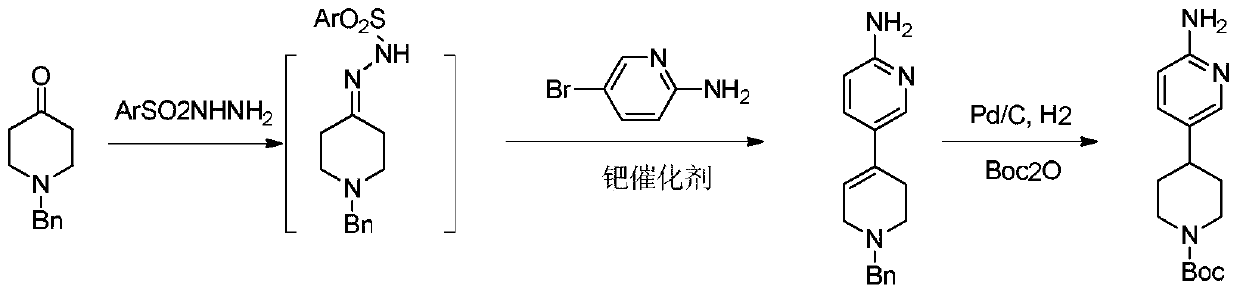
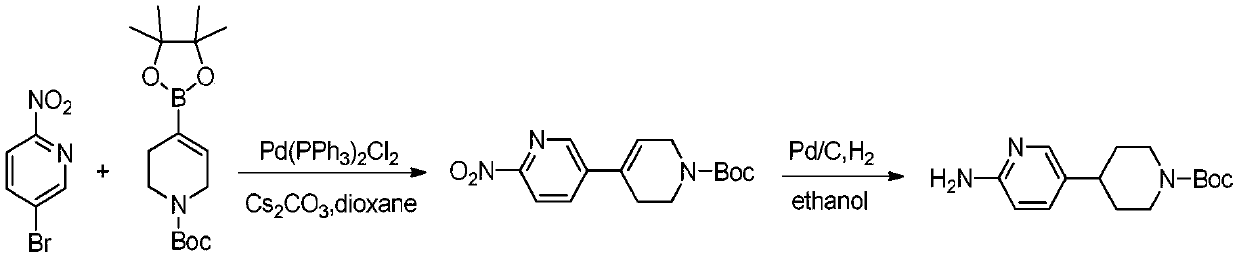
Process method suitable for amplification preparation of 4-(6-aminopyridin-3-yl) substituted piperidine
A technology of base piperidone and piperidine, which is applied in the field of amplifying the preparation of 4-substituted piperidines, can solve the problems of difficult purification and removal of impurities, low synthesis process yield, poor economic benefits and the like, and achieves short route steps and optimized preparation. Craftsmanship, universal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] Step 1: Preparation of 6-amino-3',6'-dihydro-2'-hydrogen-[3,4']bipyridyl-1'-formyl tert-butyl ester
[0043] Add N-tert-butoxycarbonyl-4-piperidone (1.45g), p-toluenesulfonyl hydrazide (1.35g), and dioxane (31.2g) into the bottle, and replace nitrogen three times under stirring conditions. The temperature was raised to 75°C and the reaction was stirred for 5h. Spot plate monitoring shows that after the point of N-tert-butoxycarbonyl-4-piperidone disappears, distill off 20 g of solvent, and under nitrogen protection, add Xphos (0.11 g), [1,1'-bis(diphenyl Phosphinoyl)ferrocene]palladium dichloride (0.029g), sodium tert-butoxide (1.34g), 2-amino-5-bromopyridine (1g), heated to 110°C for 8h, cooled, filtered, added 5ml of methyl tert-butyl ether, beat for 10 minutes, filter the obtained solid and dry it to obtain 6-amino-3',6'-dihydro-2'-hydrogen-[3,4']bipyridyl-1' - Formyl tert-butyl ester 1.30 g, purity: 99.1%, yield: 82%. 1 H-NMR (400MHz, DMSO-d 6):7.94...
Embodiment 2
[0048]
[0049] Add N-benzylpiperidone (1.38g), p-toluenesulfonyl hydrazide (1.35g), and dioxane (31.2g) into the bottle, replace nitrogen three times under stirring conditions, and heat up to 75°C and stir Reaction 5h. Spot plate monitoring showed that after the N-benzylpiperidone point disappeared, 20 g of solvent was evaporated, and under nitrogen protection, tricyclohexylphosphine (0.064 g), tris(dibenzylideneacetone) dipalladium (0.0106 g) were added successively in the bottle. g), cesium carbonate (1.34g), 2-amino-5-bromopyridine (1g), heated to 110°C for 8h, filtered to obtain 6-amino-3',6'-dihydro-2'-hydrogen-[ 3,4']bipyridyl-1'-benzyl solution, external standard content 5.1%. Add Boc anhydride (1.1g) to the reaction solution, add 5% palladium carbon (0.06g), and pump hydrogen three times under stirring. Finished with diatomaceous earth filtration and concentration to obtain a paste, beating with 20ml methyl tert-butyl ether, filtered and dried to obtain solid 4-(...
Embodiment 3
[0051]
[0052] In the reaction flask, add 1-Cbz-4-piperidone (1.70g), p-toluenesulfonyl hydrazide (1.35g), dioxane (31.2g), replace nitrogen three times under stirring conditions, and heat up The reaction was stirred at 75°C for 5h. After spot plate monitoring showed that the 1-Cbz-4-piperidone point disappeared, the solvent was evaporated and 10 g of dioxane was added. Under nitrogen protection, 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (0.13g), [1,1'-bis(diphenylphosphino)ferrocene ] Palladium dichloride dichloromethane complex (0.047g), potassium carbonate (2.3g), 2-amino-5-bromopyridine (1g), heated to 110 ° C for 8h, filtered to obtain 6-amino-3' , 6'-dihydro-2'-hydrogen-[3,4']bipyridyl-1'-CBZ solution, external standard content 5.0%, content yield: 83%. MS(m / z)310.3(M+H) + .In this solution, add isopropanol (25mL), add Boc anhydride (1.1g), add 10% palladium carbon (0.05g) under the protection of nitrogen, and replace the hydrogen three times under stirring, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com