Humanized anti-Periostin monoclonal antibody as well as preparation method and application of humanized anti-Periostin monoclonal antibody
A monoclonal antibody and humanized technology, applied in the field of medicine, can solve the problems that hinder the survival and continuous benefit of colorectal cancer patients, achieve the effects of inhibiting growth and recurrence, easy operation, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1: Preparation method of humanized anti-Periostin monoclonal antibody
[0116] The preparation method of the humanized anti-Periostin monoclonal antibody of the present embodiment comprises the following steps:
[0117] Step 1: Extract total RNA from mouse hybridoma cell LC-5A6
[0118] The mouse hybridoma cell LC-5A6 was purchased from the China General Microorganism Culture Collection and Management Center, the preservation number is CGMCCNo.10898, and it was stored in liquid nitrogen. Extract the total RNA of mouse hybridoma cell LC-5A6, operate according to the instructions of Trizol (Gibco Company), specifically: collect 1 × 10 7 Mouse monoclonal antibody hybridoma cells were centrifuged at 10,000 rpm for 1 min, and the supernatant was discarded. Add 1mL Trizol to fully dissolve the cells, let stand at room temperature for 3-5min, then add 0.2mL chloroform, mix upside down; centrifuge at 12,000rpm at 4°C for 10min, transfer the supernatant to a clean 1.5m...
Embodiment 2
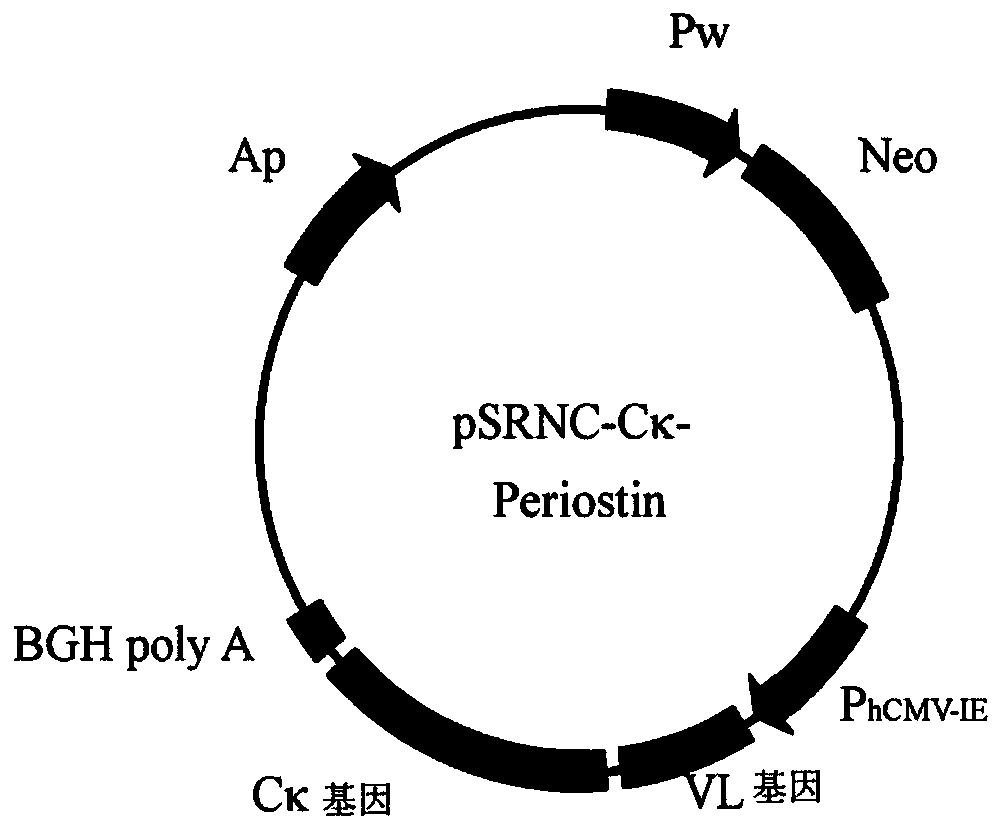
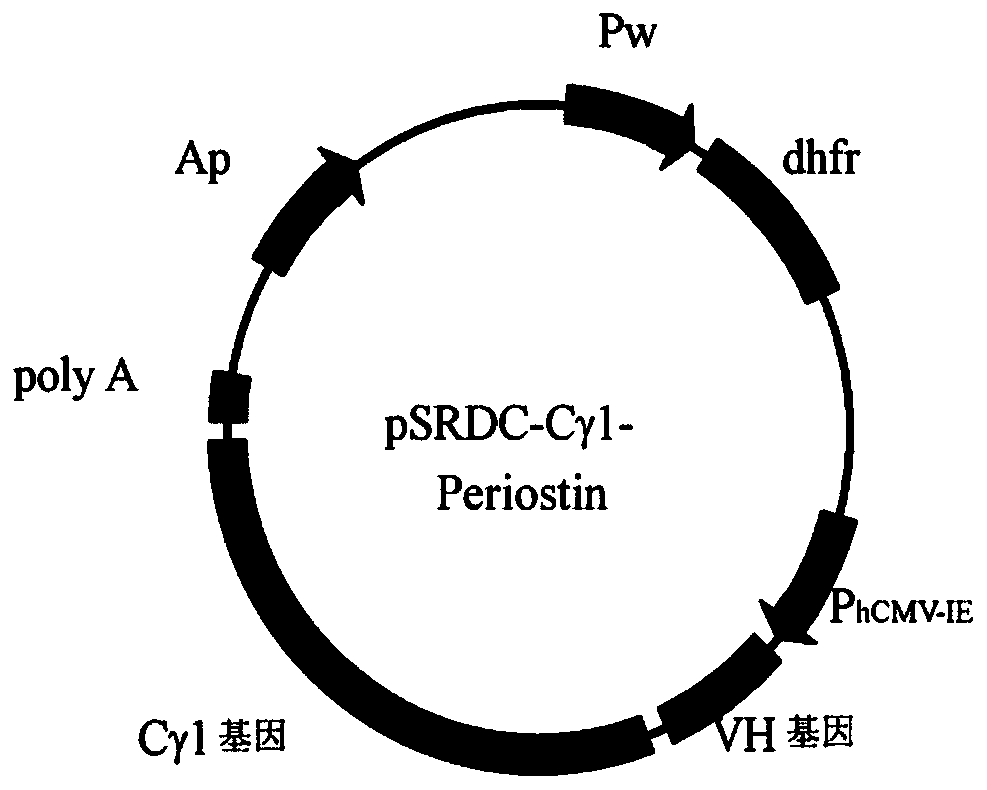
[0147] Example 2: Construction of humanized antibody gene and expression vector of anti-Periostin mouse monoclonal antibody
[0148] Using the gene synthesis method, the framework region of the variable region gene of the anti-Periostin mouse monoclonal antibody was replaced with the framework region of the human antibody, and the humanized antibody variable region gene fragment of the anti-Periostin mouse monoclonal antibody was synthesized by the total synthesis method, and recombined Into the vector containing the regulatory sequence and the human antibody constant region gene, the complete gene of the anti-Periostin humanized antibody and the eukaryotic expression vector containing the gene were constructed.
[0149] Synthesis of the humanized antibody variable region gene of the anti-Periostin mouse monoclonal antibody: using the humanized 4D5 monoclonal antibody variable region as a template, the CDR region was replaced by the corresponding 3B8 monoclonal antibody CDR reg...
Embodiment 3
[0163] Example 3: Induced Expression in Cells (CHO Cells) of Humanized Anti-Periostin Antibody Expression Vector
[0164] Will contain 10% FBS, 0.03mmol / L hypoxanthine (H), 0.003mmol / L thymidine (T), 0.1mmol / L proline (Pro), 0.1mmol / L glycine (Gly), DMEM medium with 100U / mL penicillin and streptomycin and 2mmol / L glutamine was placed in 5% CO 2 , in a 37°C environment, recover and culture CHO-dhfr - cell. According to the ratio of 1:10, subculture once every 3-4 days. Using the gene transfection method, use Lipofect AMINE reagent (Gibco Company) for transfection, co-transfect the light and heavy chain expression vectors of the anti-Periostin humanized antibody into the cells, and use medium without H, T, and Gly Screening was carried out, and after the clones were formed, selection medium containing 200 μg / mL G418 (Gibco Company) was used for screening. As a result, 4 μg of light and heavy chain antibody gene expression vectors were transfected into CHO-dhfr- cells, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com