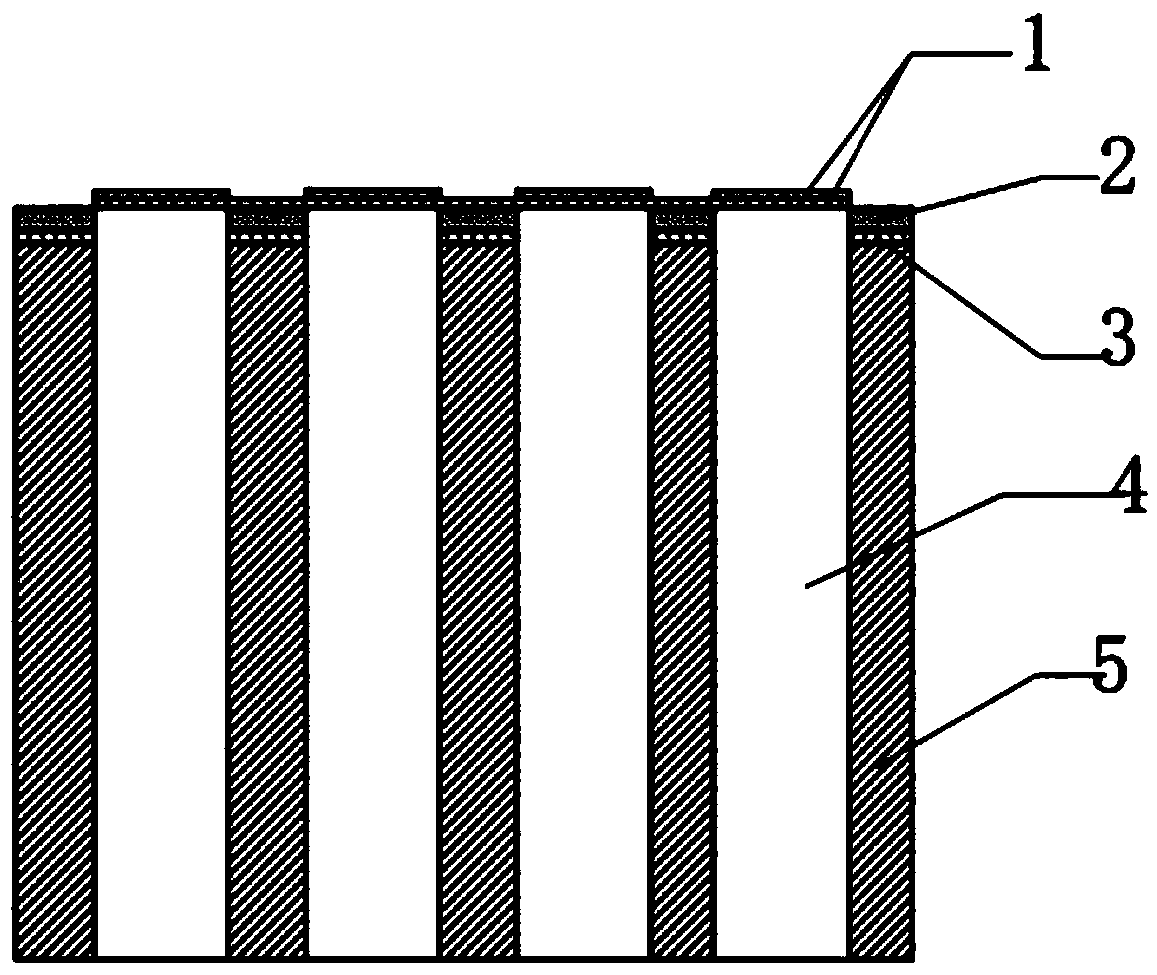
Hydrated salt phase change energy storage material and preparation method thereof, and battery thermal management system
A phase change energy storage material, hydrated salt technology, applied in secondary batteries, heat exchange materials, chemical instruments and methods, etc. Out of control and other problems, to achieve the effect of battery thermal runaway protection, improve performance and life, and improve safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
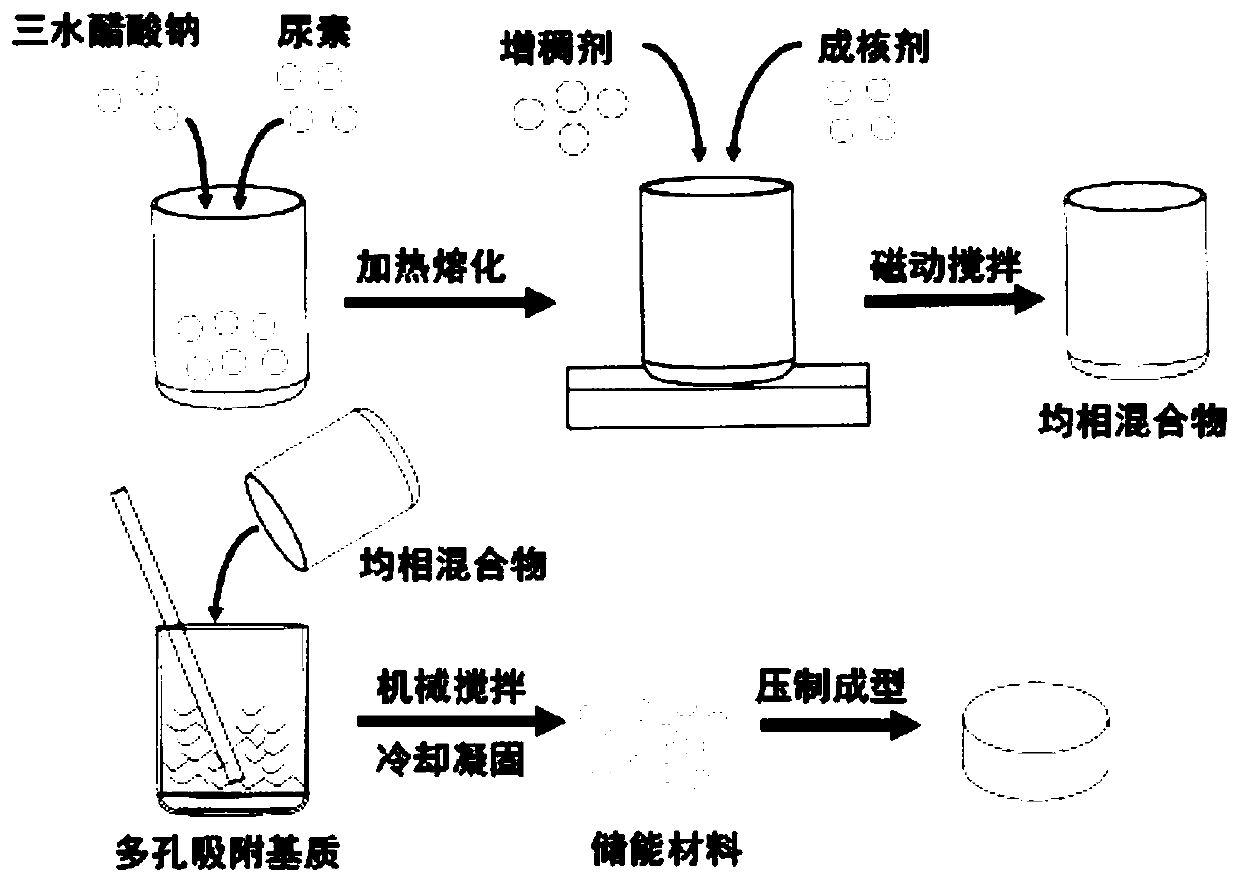
[0036] Such as figure 2 Said, a preparation method of a hydrated salt phase change energy storage material, comprising the steps of:
[0037] Mix sodium acetate trihydrate and urea at a mass ratio of 91:9, heat to 70°C to melt, stir evenly with a magnetic force, add sodium hydroxymethyl cellulose with a mass percentage of 0.5%, stir magnetically until homogeneous, and then add Graphite powder with a mass percentage of 2.5% was magnetically stirred to obtain a homogeneous mixture. The mixture is mixed and adsorbed with 50 mesh expanded graphite in a mass ratio of 80:20, and continuously mechanically stirred until the mixture is completely absorbed in the expanded graphite, sealed and cooled at room temperature to solidify, and the mass fraction is 80% acetic acid trihydrate Sodium-urea / expanded graphite composite phase change energy storage material.
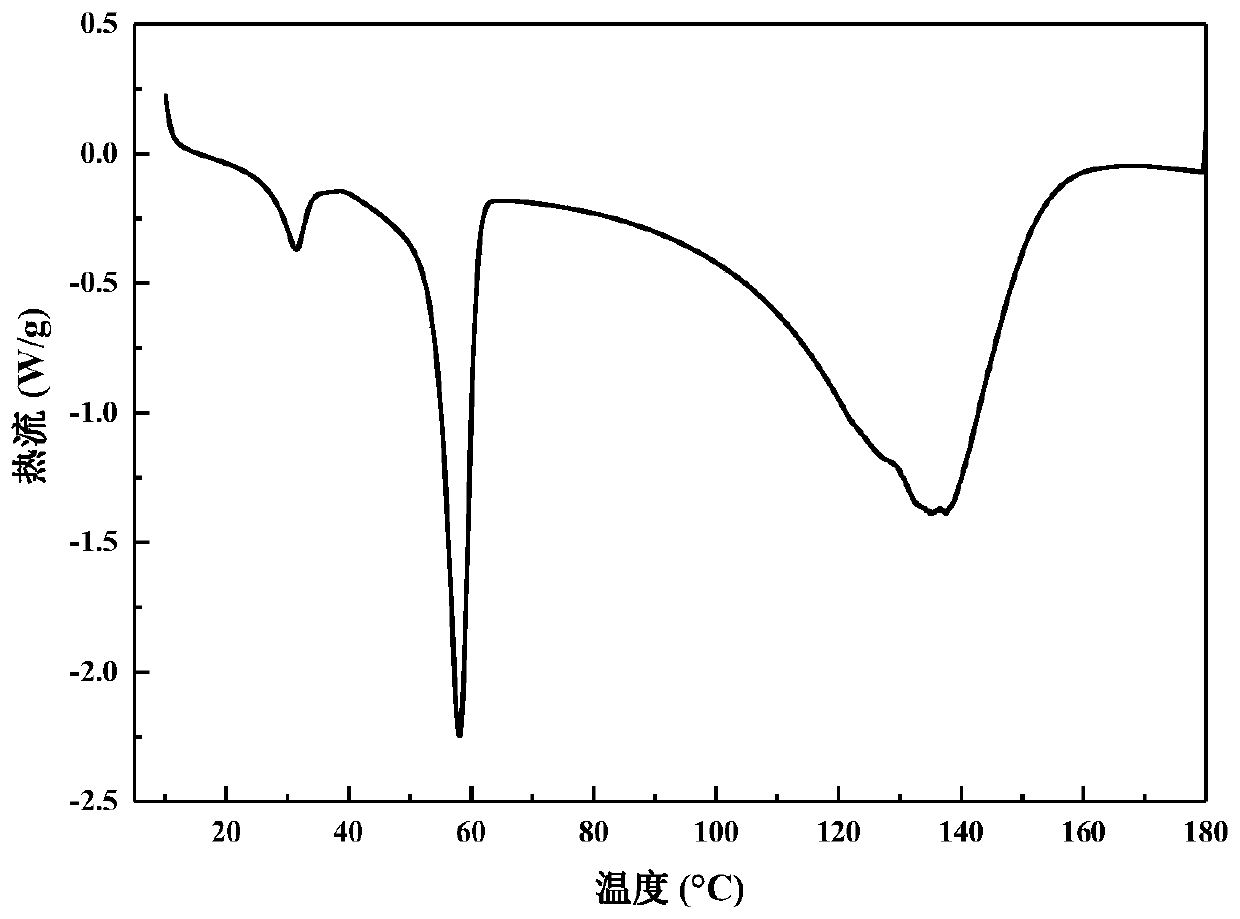
[0038] image 3 It is the DSC curve of the composite phase change energy storage material obtained in Example 1. The phase...
Embodiment 2
[0042] A preparation method of a hydrated salt phase change energy storage material, comprising the steps of:
[0043] Heat 99.0% by mass sodium acetate trihydrate to 70°C to melt, add 1.0% by mass sodium hydroxymethyl cellulose, and magnetically stir until homogeneous. The homogeneous mixture is mixed and adsorbed with 50 mesh expanded graphite at a mass ratio of 72.5: 27.5, and continuously mechanically stirred until the mixture is completely absorbed into the expanded graphite, then adding graphite powder with a mass percentage of 1.0% and mixing evenly, at room temperature After cooling and solidification, a sodium acetate trihydrate / expanded graphite composite phase change energy storage material with a mass fraction of 72.5% was obtained.
[0044] The hydrated salt composite phase change energy storage material obtained in this example has a phase transition temperature of 58.3 °C, a phase transition enthalpy of 181.0 J / g, a thermal decomposition temperature of 114.1 °C, a...
Embodiment 3
[0046] A preparation method of a hydrated salt phase change energy storage material, comprising the steps of:
[0047] Mix sodium acetate trihydrate and urea with a mass ratio of 9:1, heat to 70 ° C to melt, stir evenly with a magnetic force, add sodium hydroxymethyl cellulose with a mass percentage of 1.0%, stir magnetically until homogeneous, and then add The mass percent is 2.0% graphite powder, magnetically stirred to obtain a homogeneous mixture. The mixture is mixed and adsorbed with 50 mesh expanded graphite in a mass ratio of 75:25, and continuously mechanically stirred until the mixture is completely absorbed into the expanded graphite, sealed and cooled at room temperature to solidify to obtain acetic acid trihydrate with a mass fraction of 75%. Sodium-urea / expanded graphite composite phase change energy storage material.
[0048] The hydrated salt composite phase change energy storage material obtained in this example has a phase transition temperature of 30-55°C, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com