Genetic engineering bacterium for producing L-leucine, and application of genetic engineering bacterium
A technology of genetically engineered bacteria and leucine, applied in the field of metabolic engineering, can solve the problems of unstable fermentation, slow growth of L-leucine-producing strains, nutrient deficiencies, etc., and achieves fast growth, short fermentation period and high transformation rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
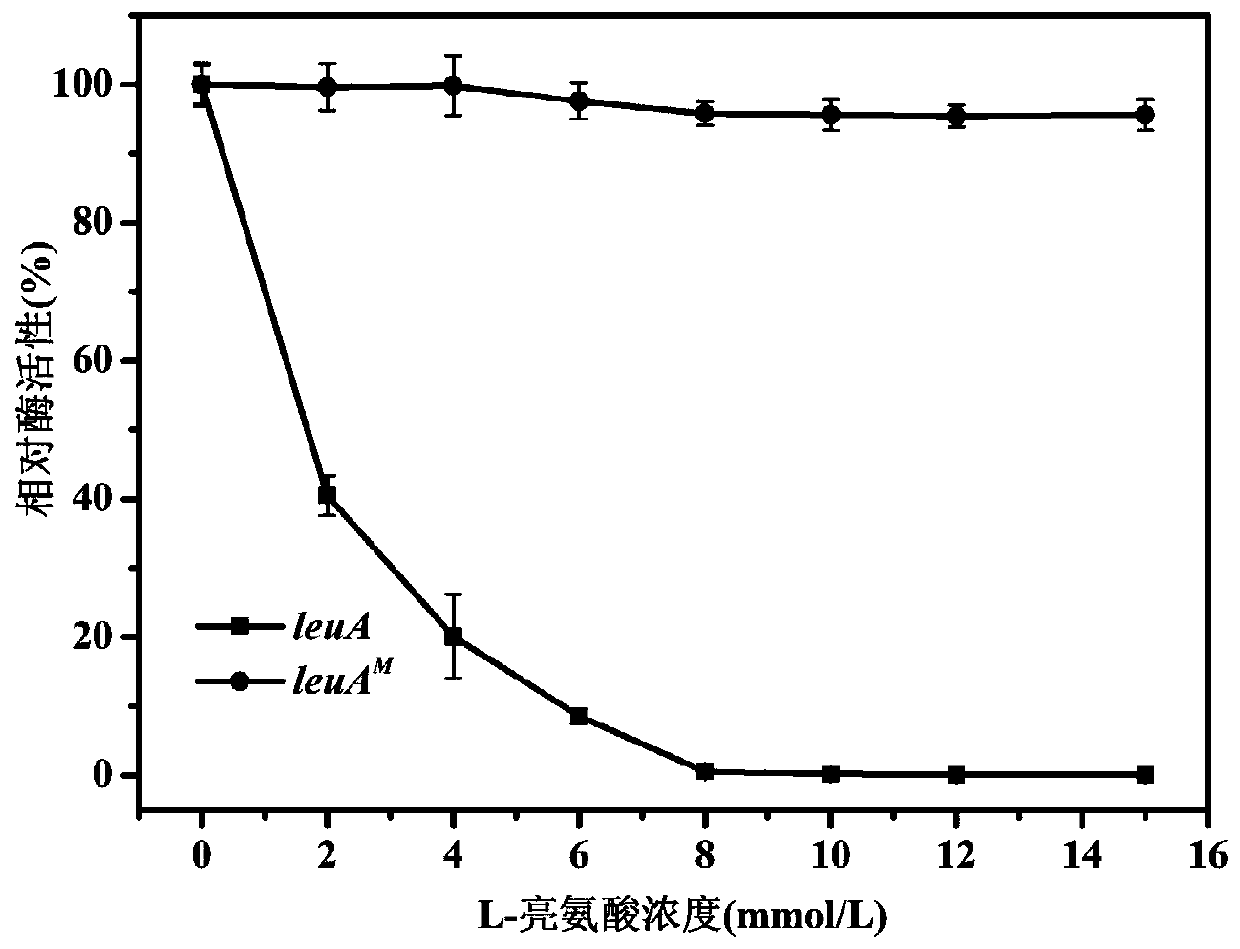
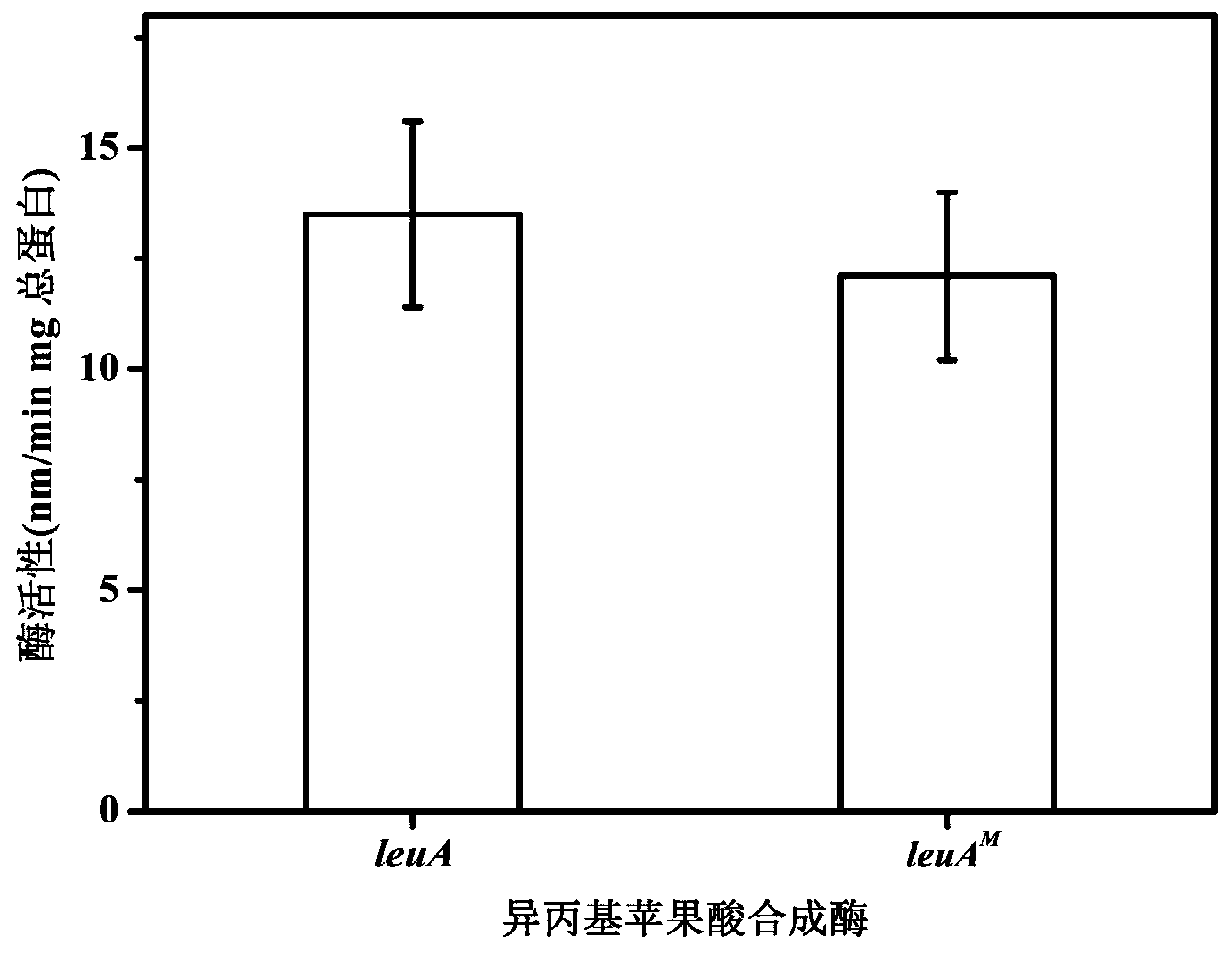
[0057] Example 1: Isopropylmalate Synthase Encoding Gene leuA Relieving L-Leucine Feedback Inhibition M the acquisition
[0058] (1) Screening of mutants resistant to L-leucine structural analogues
[0059] ① Preparation of Corynebacterium glutamicum (Corynebacterium glutamicum) ATCC13032 bacterial suspension
[0060] Corynebacterium glutamicum (Corynebacterium glutamicum) ATCC13032 was inoculated into LB liquid medium, cultured at 32°C, 200rpm for 12h, collected by centrifugation, washed with sterile saline for 3 times and then resuspended to make the OD 600 =0.6-0.8, take 10 μL of the bacterial suspension and spread it on the slide.
[0061] ② Plasma mutagenesis at atmospheric pressure and room temperature
[0062] The mutagenesis parameters were as follows: the slide was placed at 2mm from the airflow port, the power was 120W, the airflow was 10SLM, and the action time was 20s.
[0063] ③ Screening of mutant strains resistant to L-leucine structural analog α-aminobutyri...
Embodiment 2
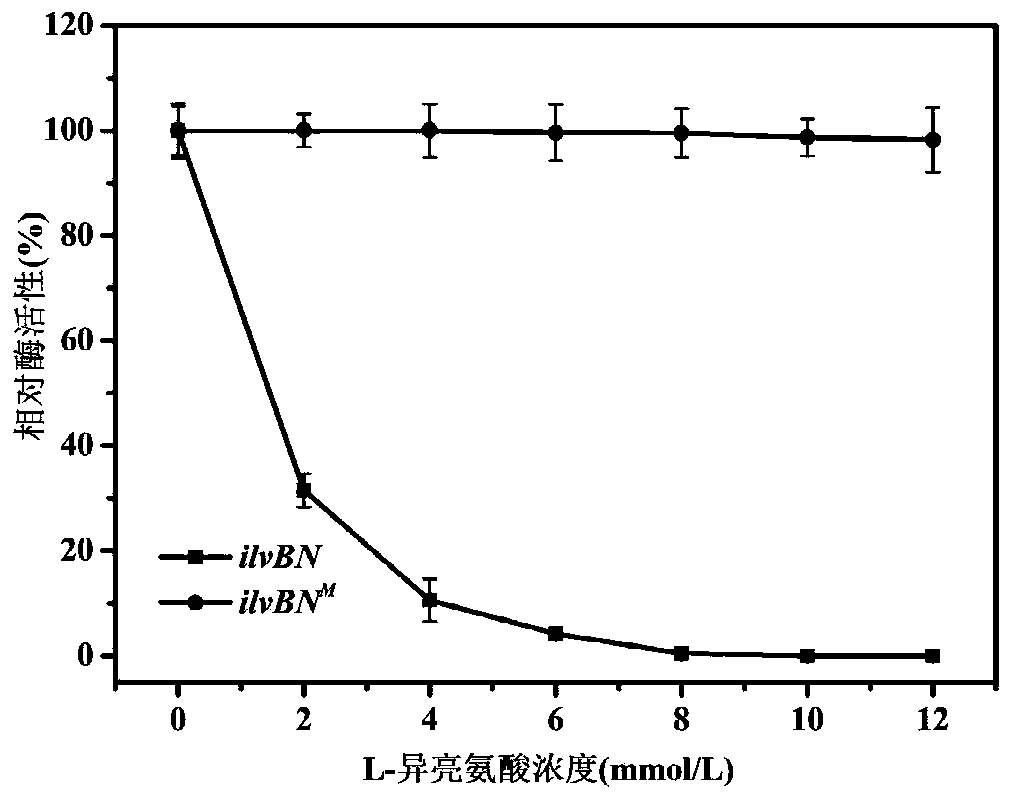
[0085] Example 2 Release the acetohydroxyacid synthase coding gene ilvBN of L-isoleucine feedback inhibition M the acquisition
[0086] (1) Screening of mutants resistant to L-isoleucine structural analogues
[0087] ① Preparation of Corynebacterium glutamicum (Corynebacterium glutamicum) ATCC13032 bacterial suspension
[0088] Corynebacterium glutamicum (Corynebacterium glutamicum) ATCC13032 was inoculated into LB liquid medium, cultured at 32°C, 200rpm for 12h, collected by centrifugation, washed with sterile saline for 3 times and then resuspended to make the OD 600 =0.6-0.8, take 10 μL of the bacterial suspension and spread it on the slide.
[0089] ② Plasma mutagenesis at atmospheric pressure and room temperature
[0090] The mutagenesis parameters were as follows: the slide was placed at 2mm from the airflow port, the power was 120W, the airflow was 10SLM, and the action time was 25s.
[0091] ③ Screening of mutant strains resistant to L-isoleucine structural analog ...
Embodiment 3
[0110] Embodiment 3: Construction of L-leucine producing bacteria TE03
[0111] (1) Recombinant fragment UHF-leuA M - Construction of DHF
[0112] synthetically containing leuA M The plasmid of the gene is used as a template, and LEUA-3 and LEUA-4 are used as primers for PCR amplification to obtain leuA M ;
[0113] Using the Escherichia coli W3110 genome as a template, using primers LEUA-1 and LEUA-2 and LEUA-5 and LEUA-6 to amplify the fragments UHF and DHF respectively, UHF and DHF are the upper and lower homology arms of the lacI gene respectively; UHF, DHF and leuA M As a template, use primers LEUA-1 and LEUA-6 for PCR amplification, and after recovery, it is the recombinant fragment UHF-leuA M -DHF.
[0114] (2) Recombinant fragment UHFA-ilvBN M - Construction of DHFB
[0115] Synthetic containing ilvBN M The plasmid of the gene is used as a template, and IlvB-3 and IlvB-4 are used as primers to perform PCR amplification to obtain ilvBN M ; Using the E. coli W3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com