Method for extracting and separating rubidium ions from salt lake brine
A salt lake brine and rubidium ion technology, applied in the field of rubidium ion separation, can solve the problems of production equipment corrosion and low rubidium ion extraction efficiency, and achieve the effect of reducing the degree of corrosion and improving extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
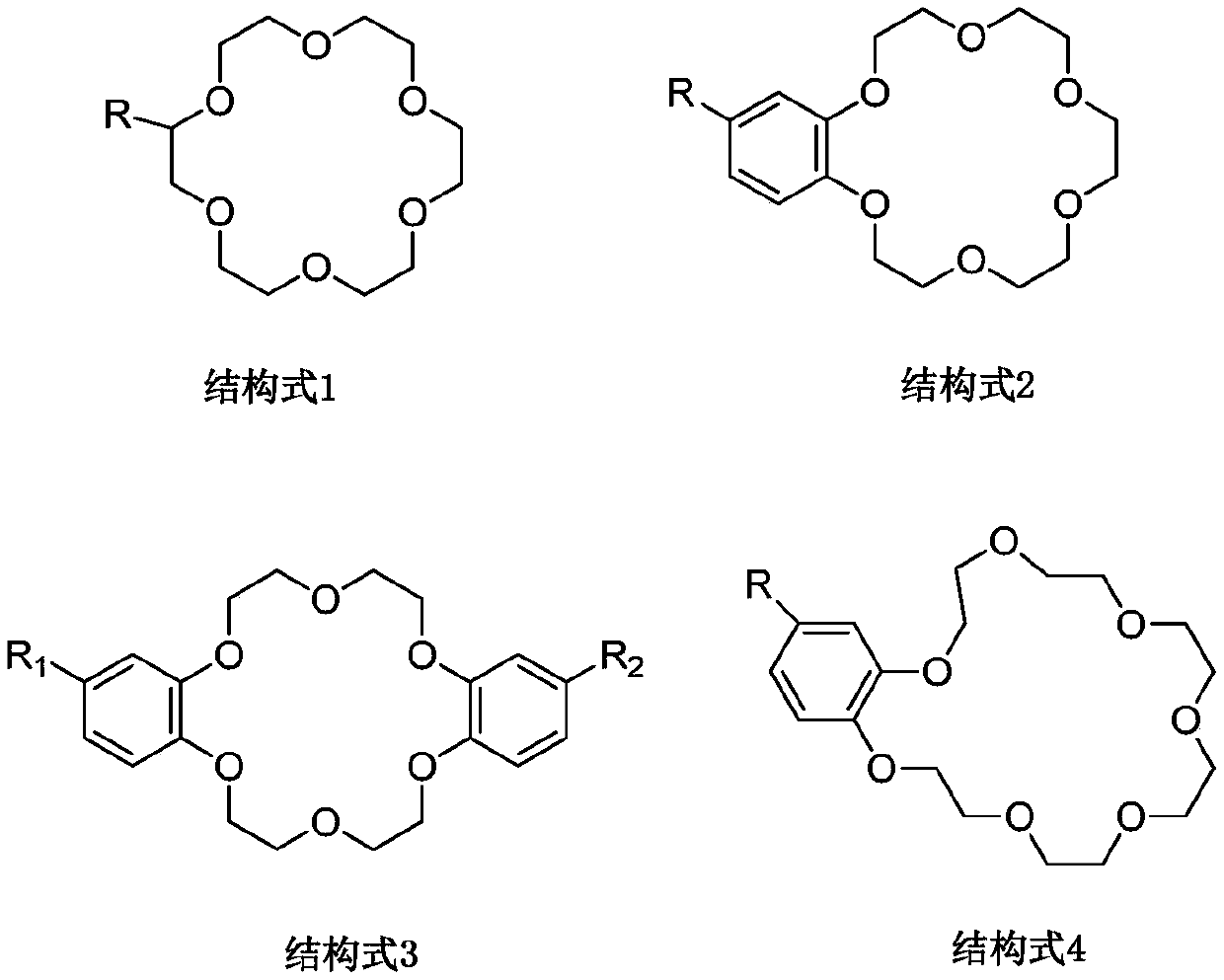
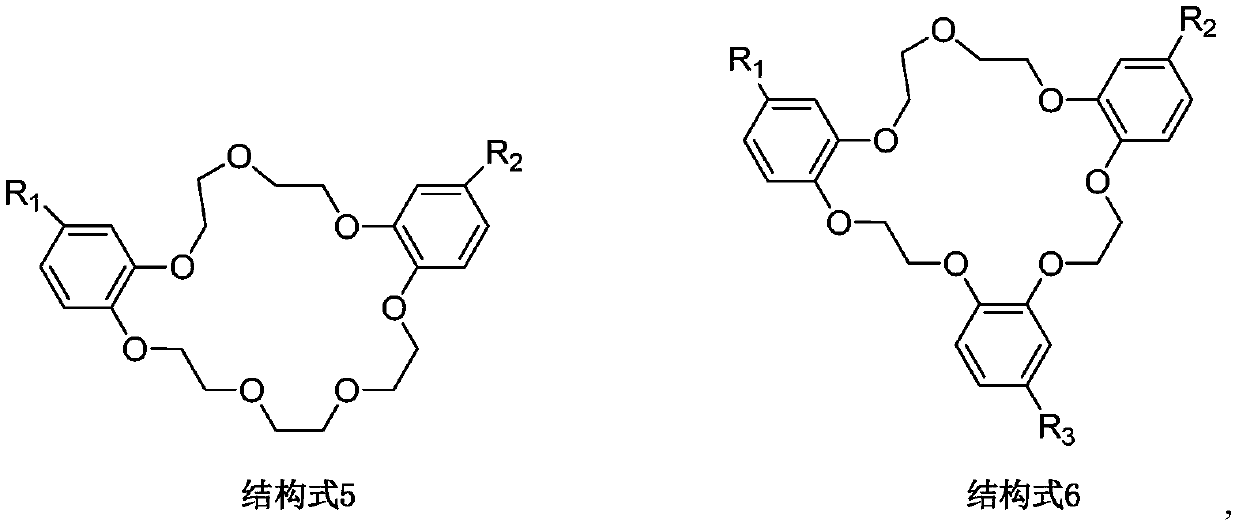
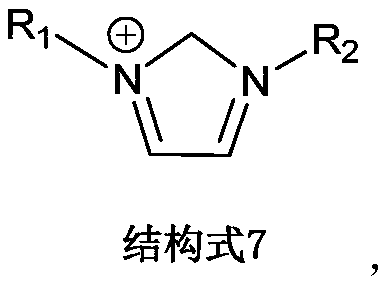
[0026] Accurately weigh 5 equal parts of extractant 18-crown-6 at room temperature, dissolve them in diluent xylene, ethyl acetate, chloroform, 1,2-chloroethane, ionic liquid 1-butyl-3 Methylimidazolium hexafluorophosphate [C 4 mim][PF 6 ], and fully stirred and mixed uniformly to form 5 different organic phases, the molar concentration of the extractant 18-crown-6 in the 5 organic phases was 0.50mol / L; the 5 different organic phases were respectively Put it in a separatory funnel with rubidium-containing brine at a volume ratio of 1:1, vibrate for 15 minutes at a speed of 300r / min, centrifuge, and separate the organic phase and the aqueous phase.
[0027] The five organic phases were tested and calculated, and the extraction rates of the five organic relative rubidium ions were 37.18%, 39.56%, 41.17%, 39.33%, and 80.21%. It can be seen that the extraction efficiency of the organic phase of rubidium ions formed by the extractant 18-crown-6 and the ionic liquid is significant...
Embodiment 2
[0029] Accurately weigh a certain amount of extractant 18-crown-6 at room temperature and dissolve in diluent 1-butyl-3 methylimidazole hexafluorophosphate [C 4 mim][PF 6 ], and fully stirred and mixed to form an organic phase, the molar concentration of the extractant 18-crown-6 in the organic phase is 0.50mol / L; the organic phase and the rubidium-containing brine are placed in a volume ratio of 3:2 In a separatory funnel, vibrate for 15 min at a rotational speed of 300 r / min, and centrifuge to separate the organic phase and the aqueous phase.
[0030] The organic phase was tested and calculated separately. The extraction rate of the five main metal ions in the brine, rubidium (Rb), potassium (K), sodium (Na), lithium (Li), and magnesium (Mg), was 82.97%. , 17.13%, 2.41%, 0.34%, 0.49%. It can be seen that the ionic liquid system has a higher single-stage extraction rate and better selectivity for rubidium ions in brine.
Embodiment 3
[0032] Accurately weigh a certain amount of extractant dibenzo-21-crown-7 at room temperature and dissolve in diluent 1-butyl-3 methylimidazole bistrifluoromethylsulfonimide salt [C 4 mim][NTf 2 ], and fully stirred and mixed uniformly to form an organic phase, the molar concentration of the extractant dibenzo-21-crown-7 in the organic phase is 0.20mol / L; the organic phase and the rubidium-containing brine are according to the volume ratio of 2: 1 Place in a separatory funnel, vibrate for 15 min at a speed of 300 r / min, and centrifuge to separate the organic phase and the aqueous phase.
[0033] The organic phase was tested and calculated, and the extraction rate of the five main metal ions in the brine, rubidium (Rb), potassium (K), sodium (Na), lithium (Li), and magnesium (Mg), was 90.88%, 20.63%, 3.35%, 0.11%, 0.13%. It can be seen that the ionic liquid system has a higher single-stage extraction rate and better selectivity for rubidium ions in brine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| extraction efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com