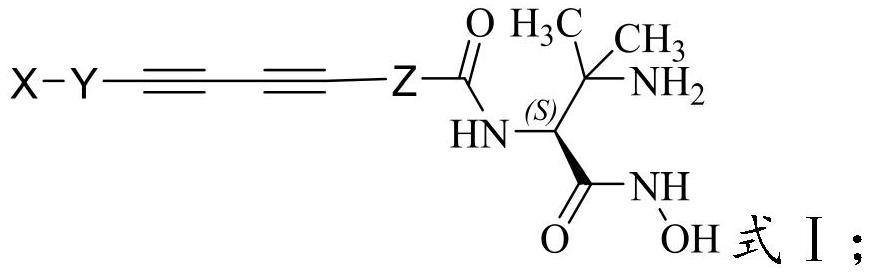
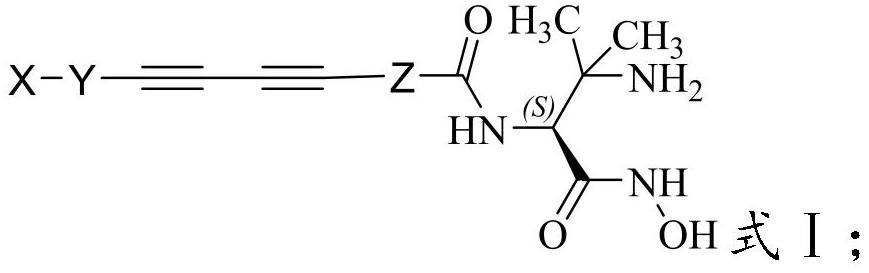
A kind of hydroxamic acid derivative and its preparation method and application
A derivative, hydroxamic acid technology, applied in the field of hydroxamic acid derivatives and its preparation, to achieve good bactericidal activity and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The present invention also provides a method for preparing the hydroxamic acid derivatives described in the above technical scheme, comprising the following steps:
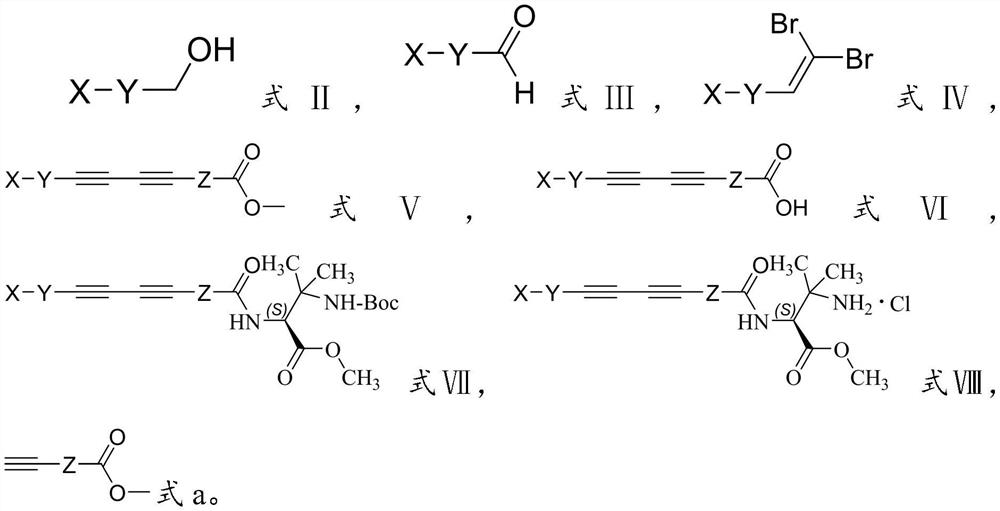
[0048] Mixing the compound with the structure shown in formula II, Dess-Martin oxidant and methylene chloride, and performing an oxidation reaction to obtain the compound with the structure shown in formula III;
[0049] Mixing the compound having the structure shown in formula III, triphenylphosphine, carbon tetrabromide and dichloromethane, performing Corey-Fuchs reaction to obtain the compound having the structure shown in formula IV;
[0050] The compound having the structure shown in formula IV, Pd 2 (dba) 3 1. Mixing a compound having a structure shown in formula a, triethylamine and N,N-dimethylformamide, performing a Sonogashira coupling reaction to obtain a compound having a structure shown in formula V;
[0051] Mixing the compound having the structure shown in formula V, tetrahydrofuran and sod...
Embodiment 1
[0120] N-((S)-3-amino-1-(hydroxylamino)-3-methyl-1-oxobut-2-yl)-4-(((1R,2S)-2-methoxycyclo Preparation of pentyl)but-1,3-diyn-1-yl)benzamide: (the preparation process is shown in formula 4)
[0121]
[0122] Preparation of (1S, 2S)-2-methoxycyclopentane-1-carbaldehyde (Ⅲ-1):
[0123] At -10°C, in a solution of II-1 (8.85g, 68mmol) in dichloromethane (250mL), add Dess Martin oxidant (DMP) (30.96g, 73mmol) in batches, and control the addition rate so that the reaction system The temperature of the mixture was kept between -7°C and -10°C, after the addition was complete, it was stirred at room temperature for 4h. After the reaction was over, move to an ice bath, add saturated aqueous sodium thiosulfate (100mL) and saturated aqueous sodium bicarbonate (250mL) successively to quench the reaction, remove the solid by filtration, leave the layers to stand, and wash the aqueous layer with dichloromethane Extract three times (100mL×3), combine the organic phases, dry over anhydrous ...
Embodiment 2
[0137] (S)-N-(3-amino-1-(hydroxyamino)-3-methyl-1-oxobut-2-yl)-4-((4-nitrophenyl)butan-1,3 -diyn-1-yl) preparation of piperazine-1-carboxamide (I-2): (preparation process is shown in formula 5)
[0138]
[0139] 4-Nitrobenzaldehyde (Ⅲ-2)
[0140] At -10°C, in a solution of II-2 (11.50g, 75mmol) in dichloromethane (250mL), add Dess Martin oxidant (DMP) (34.10g, 80mmol) in batches, and control the rate of addition so that the reaction system The temperature of the mixture was kept between -7°C and -10°C, after the addition was complete, it was stirred at room temperature for 4h. After the reaction was completed, it was moved to an ice bath, and a saturated aqueous solution of sodium thiosulfate (120 mL) and a saturated aqueous solution of sodium bicarbonate (250 mL) were sequentially added to quench the reaction. The solid was removed by filtration, the layers were left to stand, the aqueous layer was extracted three times with dichloromethane (120mL×3), the organic phases ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com