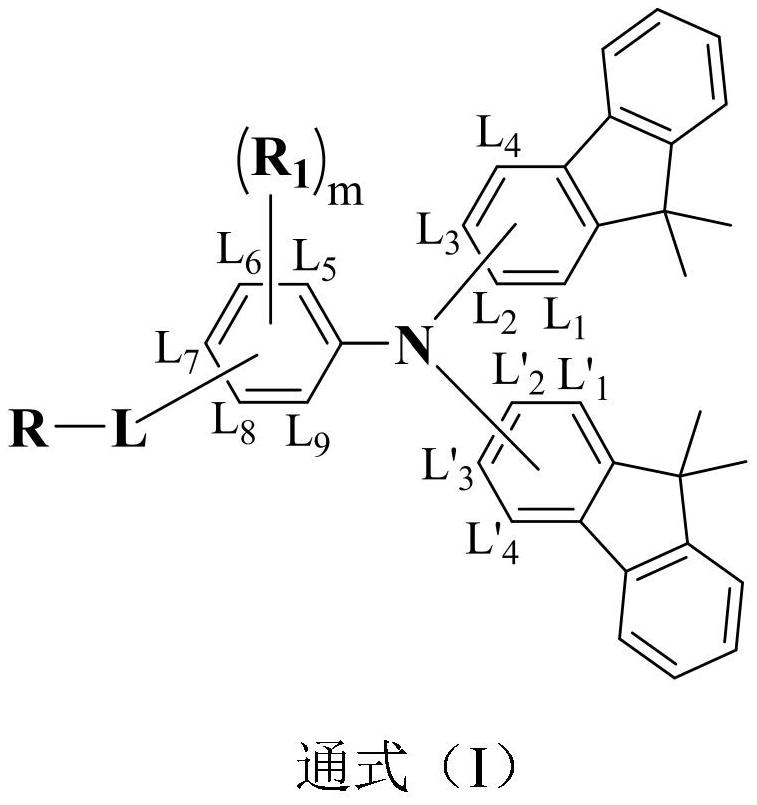
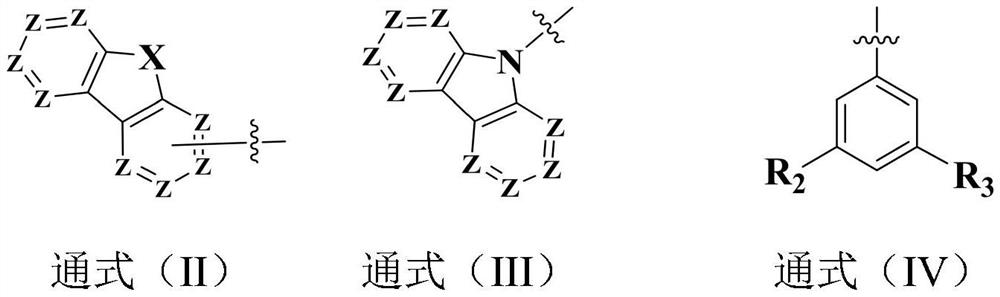
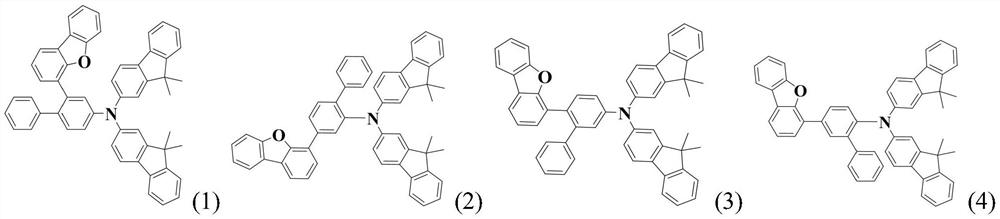
A compound based on bis-dimethylfluorene-substituted aniline and its prepared organic electroluminescent device
An electroluminescent device, bis-dimethylfluorene technology, applied in the field of semiconductors, can solve the problems of different performance and other problems, and achieve the effect of not easy to aggregate, not easy to crystallize, and polymer thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Preparation of Intermediate C-1
[0047]
[0048] Under nitrogen atmosphere, in the three-necked flask of 500ml, add 0.05mol raw material A-1, 0.05mol raw material B-1, add mixed solvent (300ml toluene, 90ml H 2 O) it was dissolved, stirred for 1 hour under nitrogen, then added 0.1mol K 2 CO 3 , 0.005mol Pd(PPh 3 )4 , heated to 90° C., reacted for 8 hours, and observed the reaction by thin layer chromatography (TLC) until the reaction was complete. After naturally cooling to room temperature, water was added to the reaction system for extraction, liquid separation, and the organic phase was rotary evaporated under reduced pressure until there was no distillate. The obtained substance was purified by silica gel column to obtain intermediate C-1 with a purity of 99.4% and a yield of 87%.
[0049] Elemental analysis structure (molecular formula C 24 h 15 BrO): Theoretical C, 72.19; H, 3.79; Br, 20.01; O, 4.01; Found: C, 72.16; H, 3.77; Br, 20.04; O, 4.03.
[0050...
Embodiment 1
[0121] Embodiment 1: the synthesis of compound 3
[0122]
[0123] Under a nitrogen atmosphere, add 0.005 mol of the prepared intermediate C-1, 0.006 mol of raw material D, 0.01 mol of sodium tert-butoxide, 3×10 -4 molPd(dba) 2 and 1.2×10 -3 mol of tri-tert-butylphosphine, then add 60ml of toluene to dissolve it, heat to reflux, react for 4 hours, and observe the reaction by TLC until the reaction is complete. Naturally cooled to room temperature, water was added to the reaction system for extraction, liquid separation, and the organic phase was rotary evaporated under reduced pressure until there was no fraction. The obtained substance was purified by silica gel column to obtain the title target product with a purity of 99.7% and a yield of 81%.
[0124] Elemental analysis structure (molecular formula C 54 h 41 NO): Theoretical C, 90.09; H, 5.74; N, 1.95; O, 2.22; Found: C, 90.06; H, 5.72; N, 1.98;
[0125] ESI-MS(m / z)(M + ): The theoretical value is 719.93, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com