A method for screening non-integrating attenuated Listeria strains highly expressing foreign proteins
An exogenous protein, Listeria technology, applied in the fields of resistance to vector-borne diseases, instruments, and biological material analysis, can solve the differential expression between Listeria colonies, unfavorable rapid rough screening of large-scale samples, and complex preparation operations. and other problems, to achieve the effect of prominent effect, convenient operation and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Example 1: Solid and liquid media, antibiotic use and non-integrating attenuated Listeria monocytogenes Sterile culture system and strain preservation
[0113] Liquid culture medium BHI (brain heart infusion broth): 74g / L, natural pH value, sterilized at 121°C for 20min.
[0114] Solid medium BHI: On the basis of liquid BHI, add 1.5g / L agar powder. After sterilization at 121°C for 20min, when the temperature dropped to about 50°C, antibiotics (chloramphenicol resistance) were added, shaken well, and the plate was poured.
[0115] Chloramphenicol (cm): The stock solution (1000×) was prepared with absolute ethanol at 25-34 mg / ml, filtered through a 0.22 μm filter membrane, and stored at -20°C. Use a final concentration of 25-34 μg / ml. Be careful to avoid light.
[0116] Culture system: about 10 7 CFU Listeria initial culture (such as LM-OVA 28 ) into 5ml of liquid BHI medium containing chloramphenicol resistance, shaker at 230rpm and 37°C for 14-16h, that is, the...
Embodiment 2
[0119] Example 2: Preparation of Non-Integrated Attenuated Listeria Vaccine and Determination of CFU Concentration
[0120] will contain about 10 7 The initial culture of Listeria monocytogenes in CFU was added to 10ml of liquid BHI medium containing chloramphenicol resistance, shaken at 230rpm and 37°C for 14-16h, centrifuged at 4500rpm for 15-20min to collect bacteria, and washed with equal volume of PBS. The cells were resuspended twice with 1 / 10 volume of PBS (containing 7% DMSO), aliquoted, and stored at -80°C.
[0121] Statistical method of CFU: Dilute the cells with PBS or medium according to a 10-fold gradient. When the gradient is diluted, it can only be diluted from the previous gradient to the next gradient, and mix well by shaking. Take 10 respectively -5 , 10 -6 , 10 -7 , 10 -8 Each concentration of 100ul was coated on BHI plates, and the number of colonies was counted. eg 10 -8 The number of colonies grown at the concentration is N, then CFU=N x 10x 10 ...
Embodiment 3
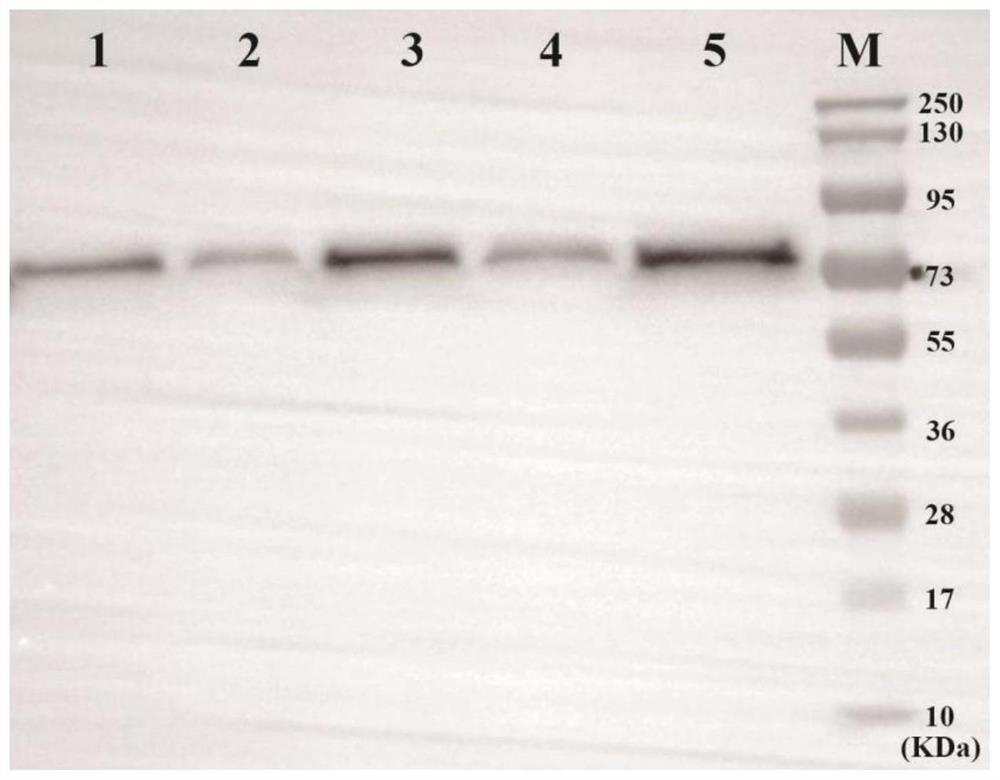
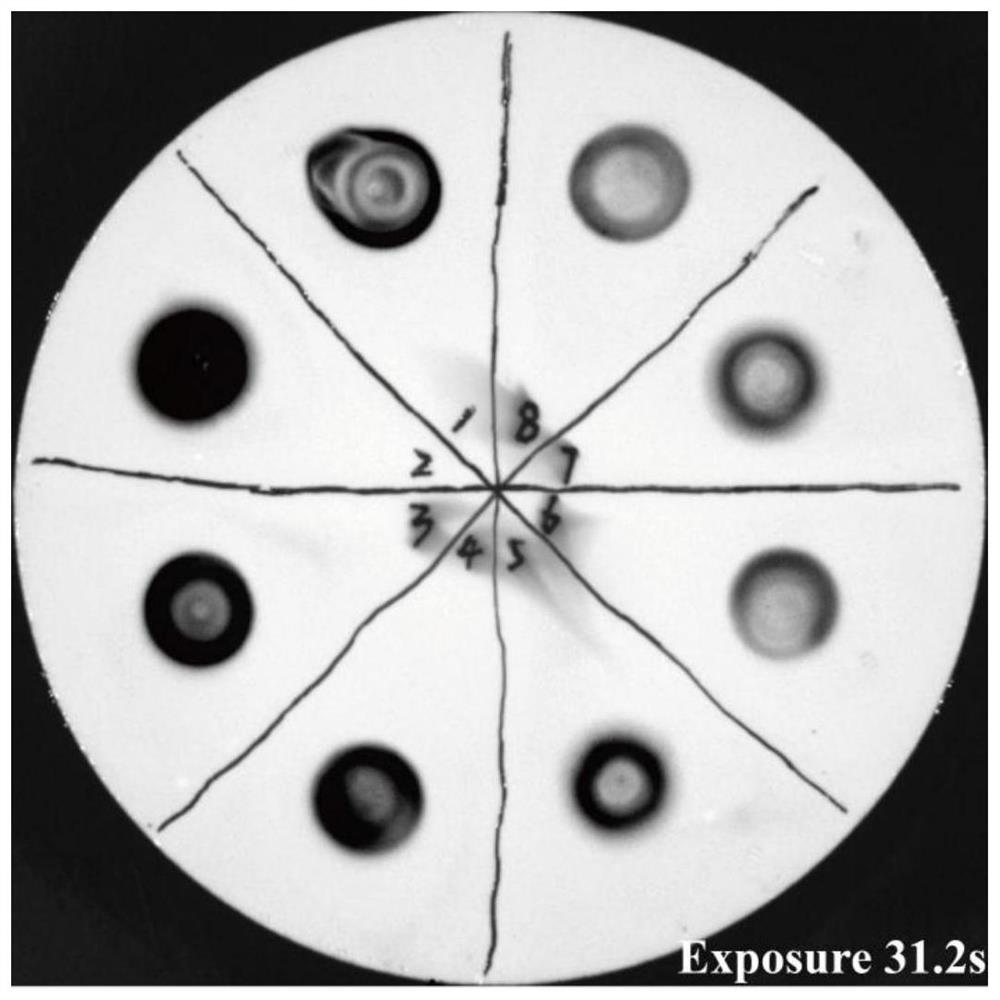
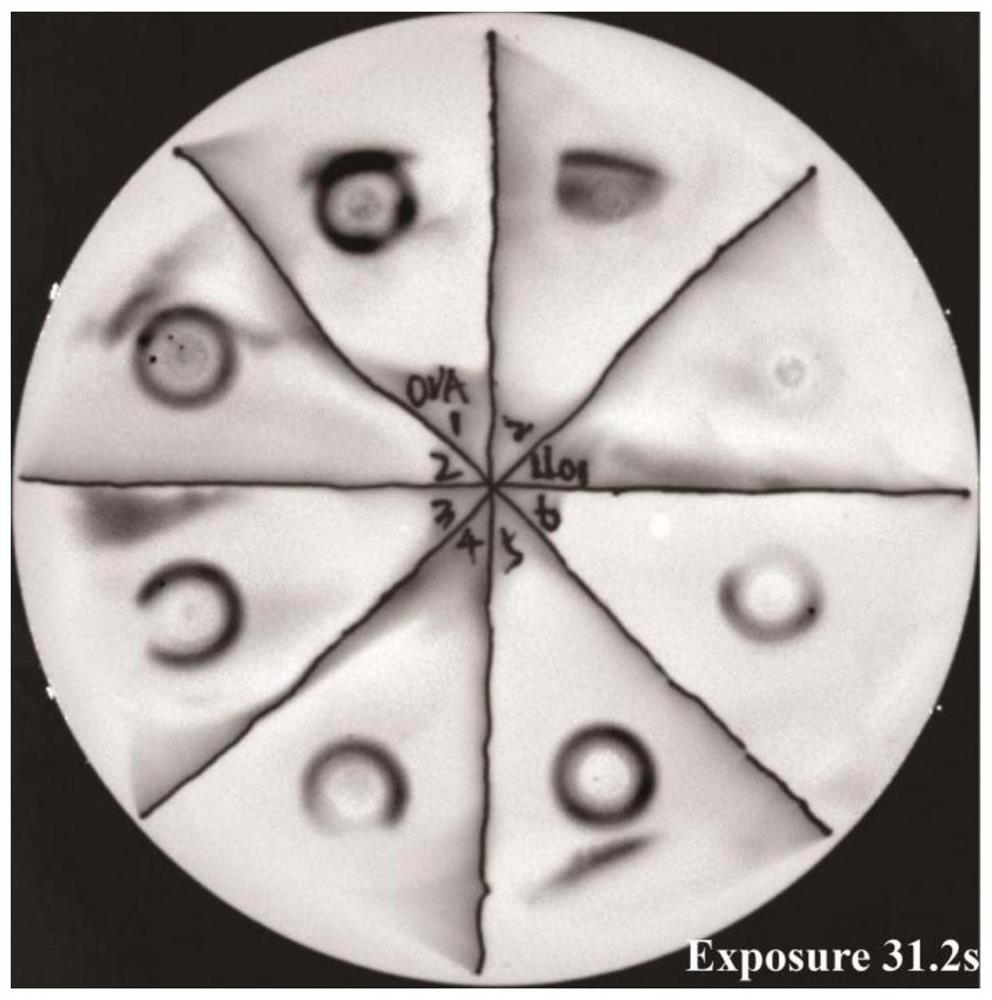
[0122] Example 3: Detection of exogenous protein expression secreted in the supernatant of non-integrating attenuated Listeria using the method of the present disclosure amount
[0123] Pick a single colony on the plate for culturing Listeria, add it to 10 ml of liquid BHI medium containing chloramphenicol resistance, shake at 230 rpm, 37 °C for 14-16 h, and centrifuge at 4500 rpm for 15-20 min to precipitate the bacteria. Take 1 ml of supernatant and mix it with 3 times the volume of 10% TCA / acetone solution, and precipitate at -20°C overnight. The precipitated proteins were collected by centrifugation at 15,000 rpm for 30 minutes, and washed twice with ice-cold acetone to remove residual TCA. The excess acetone was evaporated in a fume hood, and the pellet was dissolved in 30 μl of protein loading buffer containing 0.01 N NaOH. After boiling and denaturation, 30 μl of the sample was spotted on the NC membrane, air-dried, and washed three times with TBST, 5 min each time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com