A kind of non-integrated listeria vaccine and anti-tumor immune response method
A Listeria and vaccine technology, applied in anti-tumor drugs, bacterial antigen components, tumor-specific antigens, etc., can solve the problem of expression stability of antigen peptide expression, poor efficacy of Listeria vaccine, complex integration screening process, etc. problem, to achieve the effect of convenient operation, good expression of secreted protein, and high insertion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Embodiment 1: Construction of the plasmid of attenuated listeria
[0128] Attenuated Listeria is used in the present disclosure as a carrier strain for vaccine preparation. Exemplary, what the present disclosure is used as the bacterial strain of vaccine adopts Lm 10403SΔactA (the construction method of aforementioned bacterial strain can exemplarily refer to the following literature: Shen H et.al., PNAS, 92 (9): 3987-91, 1995 ), the bacterium lacks the actA gene, so that the bacterium infecting the host cell cannot spread to adjacent cells through its unique actin tail, thereby greatly reducing its toxicity and pathogenicity. Compared with the wild-type strain Lm10403S (LD 50 1x10 4 ), LD of Lm-ΔactA 50 0.5-1x10 8 , proved to be highly attenuated. At the same time, the bacterium retains the ability of complete LLO to escape from lysosomes, enters the cytoplasm of host cells and proliferates rapidly, and expresses proteins to activate specific T cell immune respo...
Embodiment 2
[0138] Example 2: Construction of plasmids for attenuated Listeria for vaccines
[0139] To construct the Listeria vaccine plasmid, the antigen gene needs to be inserted into the plasmid vector, which has been designed with restriction sites, and the gene sequence of the target antigen is synthesized after the company optimizes the gene codon.
[0140] Optional, OVA 28 The codon optimization process is as follows:
[0141] Mouse OVA before corresponding codon optimization 28 Nucleotide sequence (SEQ ID NO: 7):
[0142] GATGAAGTCTCAGGCCTTGAGCAGCTTGAGAGTATAATCAACTTTGAAAAACTGACTGAATGGACCAGTTCTAATGTTATGGAA
[0143] OVA after corresponding codon optimization 28 Nucleotide sequence (SEQ ID NO:8):
[0144] GATGAAGTGAGCGGCCTGGAGCAGCTGGAGAGCATTATCAACTTCGAAAAACTGACCGAGTGGACCAGCAGCAATGTGATGGAA
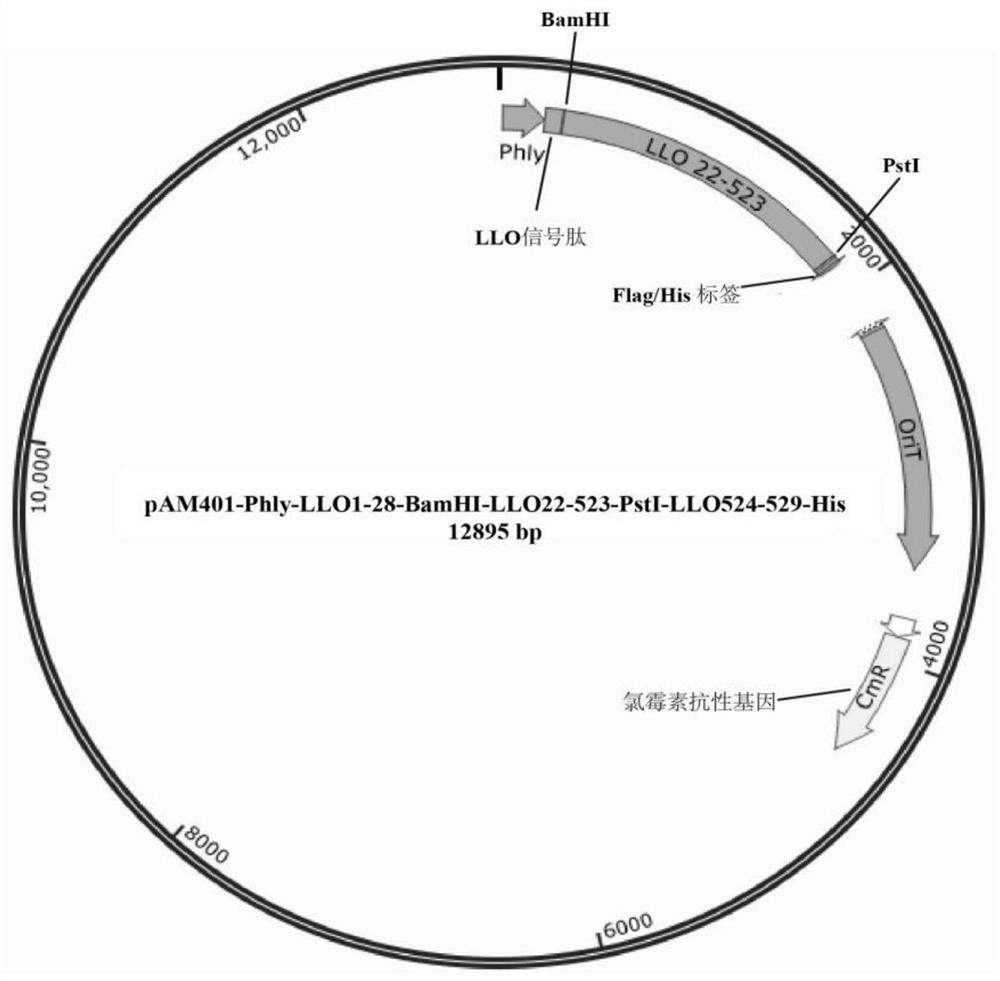
[0145] The product was cloned into pAM401-phly-LLO using homologous recombination technology based on certain homologous sequences 1-28 -BamHI-LLO 22-523 -PstI-LLO 524-540 -PstI site o...
Embodiment 3
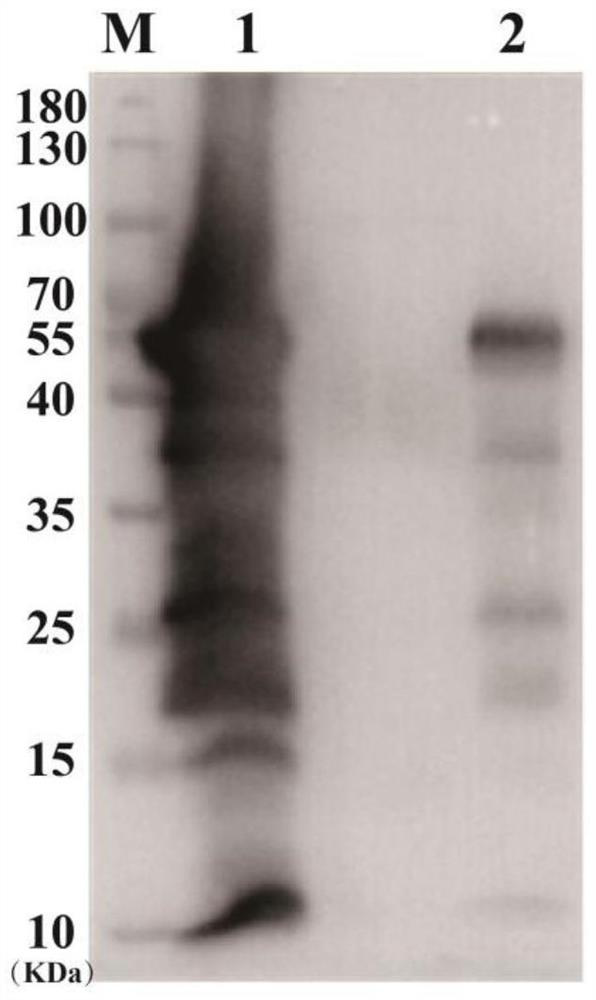
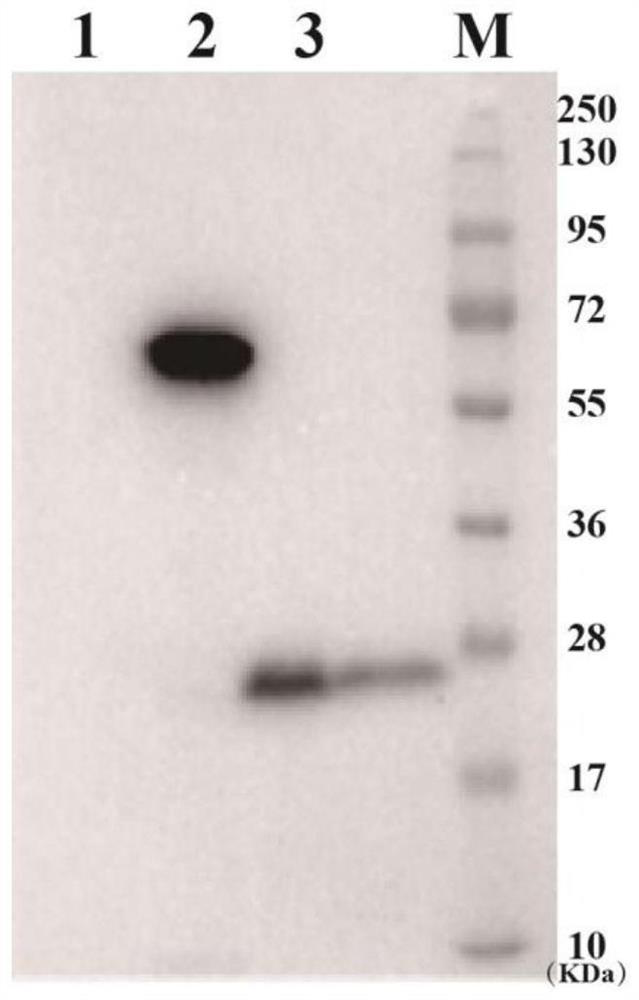
[0158] Example 3: Preparation of attenuated Listeria vaccine
[0159] The plasmids of the attenuated Listeria used for vaccines verified by sequencing were transformed into attenuated Listeria strains by electroporation technology, and single clones were selected for subsequent plasmid and expression verification.
[0160] The specific steps of the above-mentioned electrotransformation are as follows:
[0161] (1) Preparation of electroporation competent state
[0162] (i) The overnight cultured Listeria was transferred to 100-250ml brain-heart infusion broth (BHI) at a ratio of 1:50-1:200, and cultured with shaking at 37°C until OD 600 Value 0.2-0.25;
[0163] (ii) Add penicillin (PNG) to a final concentration of 10 μg / ml, and continue culturing for about two hours;
[0164] (iii) collect bacteria by high-speed centrifugation at 4°C for 5-10 minutes;
[0165] (iv) resuspend the bacterium with 200ml 10% glycerin, wash twice;
[0166] (v) Resuspend the bacteria with 45ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com