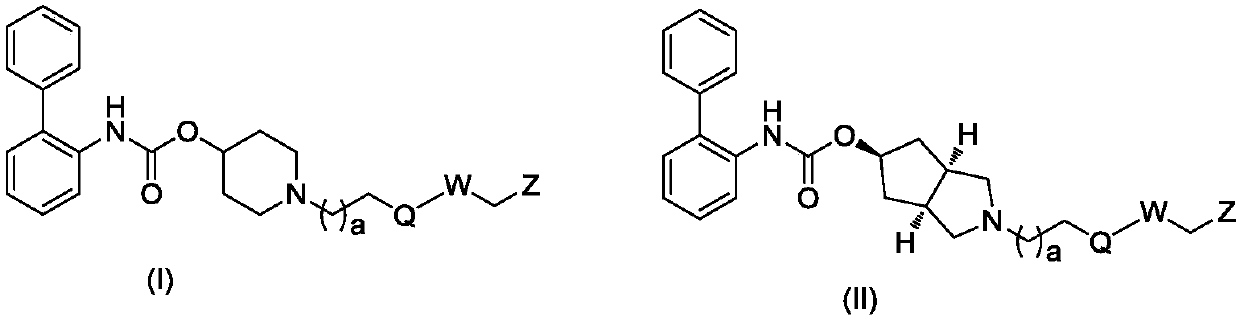
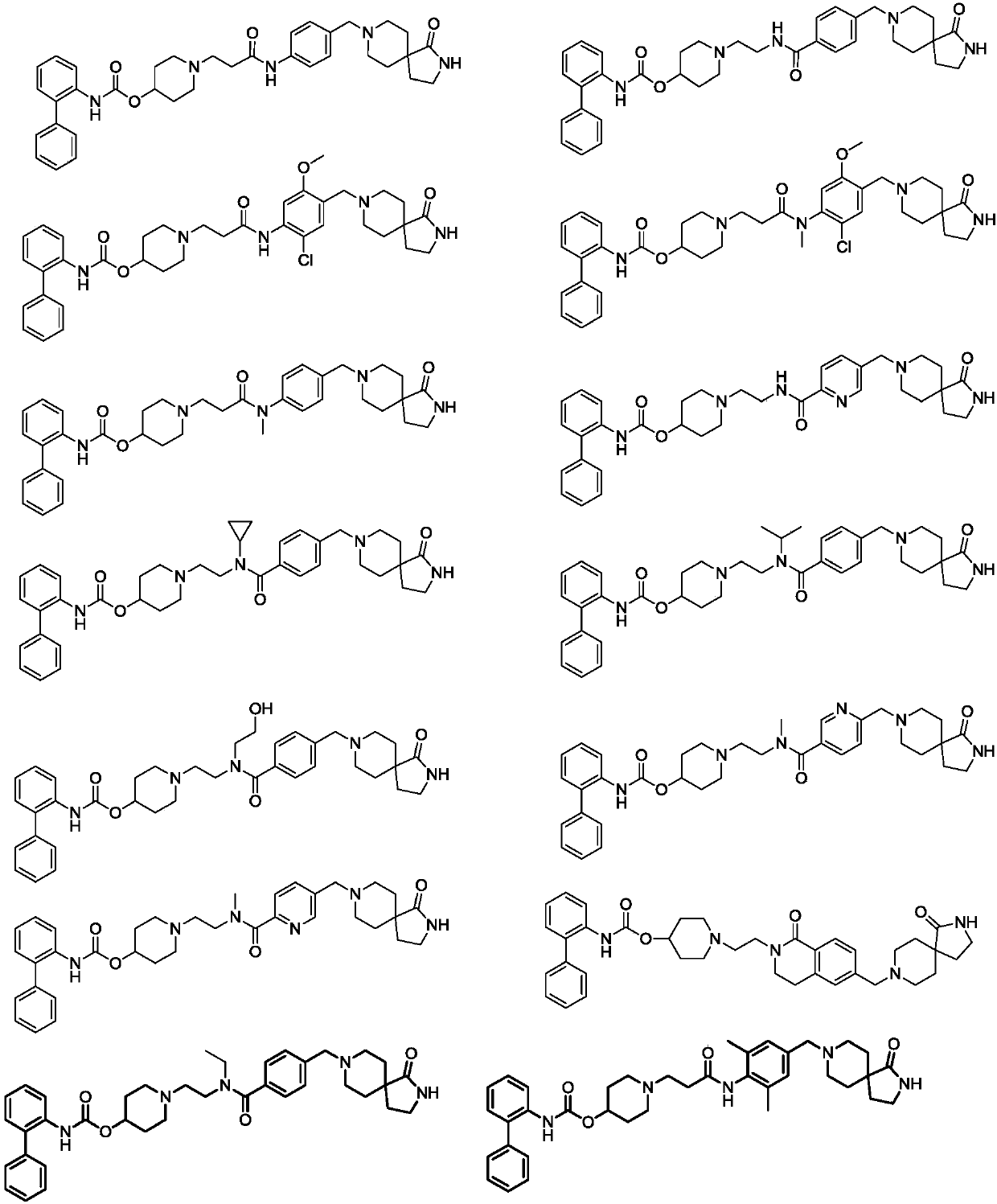
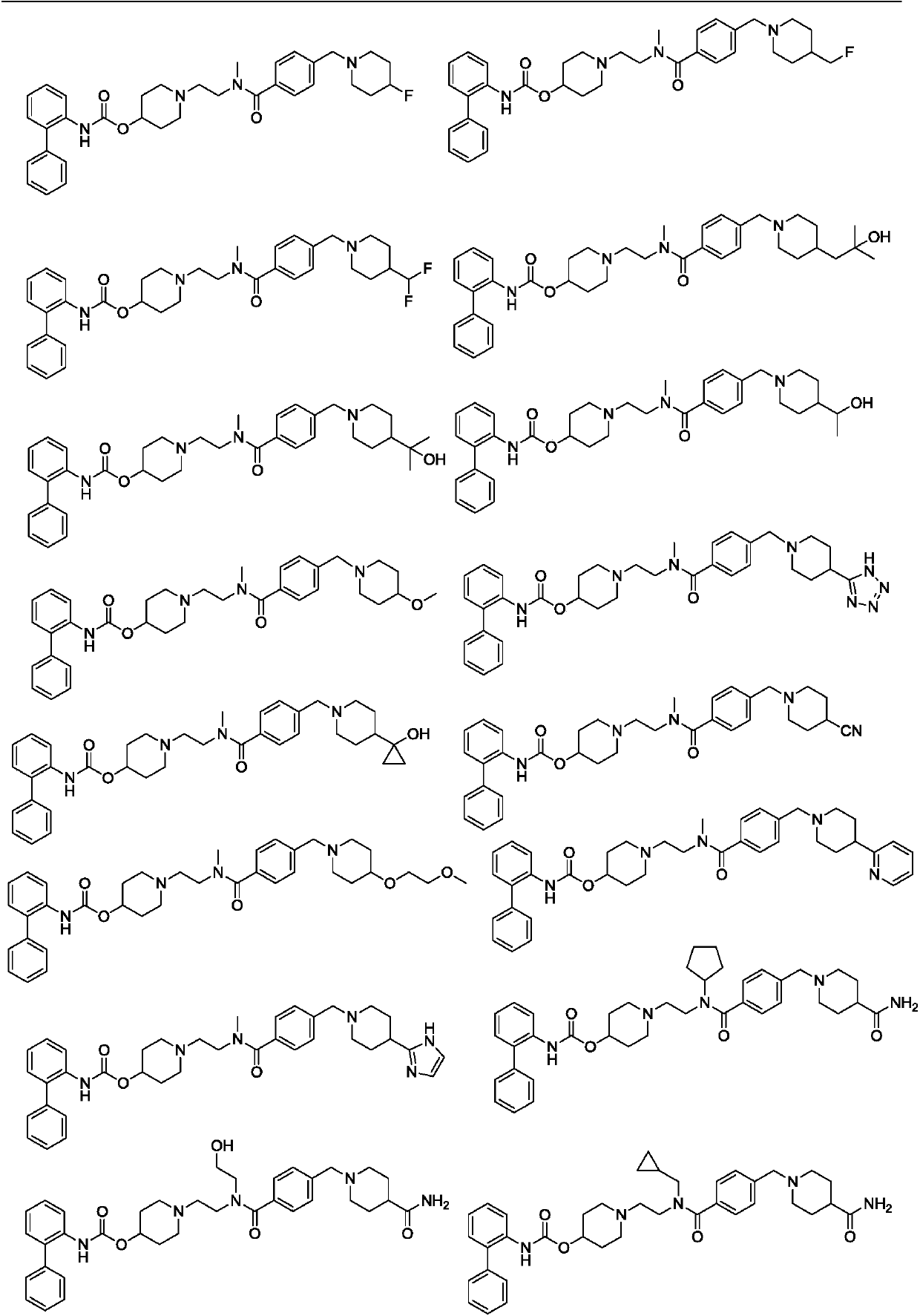
Biphenyl derivative, preparation method thereof, and application of biphenyl derivative to medicine
A pharmacy and prodrug technology, applied in the field of biphenyl derivatives and their preparation, can solve the problems of inappropriate use of patients, insufficient treatment, aggravation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Example 1: [1-[3-[4-[(2-carbamoyl-3-methyl-4,6-dihydropyrrole[3,4-d]imidazol-5-yl)methyl]- N-Methyl-anilino]-3-oxo-propyl]-4-piperidinyl]N-(2-phenylphenyl)carbamate; bistrifluoroacetate (compound 1)
[0190] [1-[3-[4-[(2-carbamoyl-3-methyl-4,6-dihydropyrrolo[3,4-d]imidazol-5-yl)methyl]-N-methyl-anilino]-3- oxo-propyl]-4-piperidyl]N-(2-phenylphenyl)carbamate; ditrifluoroacetic acidate
[0191]
[0192] Take [1-[3-(4-formyl-N-methyl-aniline)-3-oxo-propyl]-4-piperidinyl]N-(2-phenylphenyl)carbamate (1A) (0.49g, 1.0mmol) and 1-methyl-5,6-dihydro-4H-pyrrole [3,4-d] imidazole-2-carboxamide (1B) (0.33g, 2.0mmol) dissolved Add methanol (30 mL), heat to 60°C, stir until the solution is clear, cool to room temperature, add sodium triacetoxyborohydride (0.63 g, 3 mmol), and react at room temperature for 3 hours. After the reaction was completed, the reaction was quenched with saturated aqueous sodium bicarbonate (30 mL), extracted with dichloromethane (60 mL×2), dried over an...
Embodiment 2
[0194] Example 2: [1-[2-[[4-[[4-(difluoromethyl)-1-piperidinyl]methyl]benzoyl]-methyl-amino]ethyl 1]-4 -piperidinyl]N-(2-phenylphenyl)carbamate; bistrifluoroacetate (compound 2)
[0195] [1-[2-[[4-[[4-(difluoromethyl)-1-piperidyl]methyl]benzoyl]-methyl-amino]ethyl]-4-piperidyl]N-(2-phenylphenyl)carbamate; ditrifluoroacetic acidate
[0196]
[0197] [1-[2-[(4-Formylbenzoyl)-methyl-amino]ethyl]-4-piperidinyl]N-(2-phenylphenyl)carbamate (2A) (prepared with reference to WO2012009166, Example 3) (0.486g, 1.00mmol) was dissolved in dichloromethane (10ml), and 4-difluoromethylpiperidine trifluoroacetate 2A (0.257g, 1.50mmol) was added (according to patent WO2010138490) and triethylamine (0.087g), after stirring at room temperature for 1 hour, acetic acid (0.636g, 3.0mmol) was added. After stirring at room temperature for 3 hours, sodium triacetoxyborohydride (0.496 g, 2.34 mmol) was added, and the reaction was terminated after stirring overnight at room temperature. Concentrate...
Embodiment 3
[0200] Example 3: [1-[2-[[4-[(4 fluoro-1-piperidinyl)methyl]benzoyl]-methyl-amino]ethyl]-4-piperidinyl]N- (2-Phenylphenyl)carbamate (compound 3)
[0201] [1-[2-[[4-[(4-fluoro-1-piperidyl)methyl]benzoyl]-methyl-amino]ethyl]-4-piperidyl]N-(2-phenylphenyl)carbamate
[0202]
[0203] [1-[2-[(4-Formylbenzoyl)-methyl-amino]ethyl]-4-piperidinyl]N-(2-phenylphenyl)carbamate (2A) (0.486g, 1.00mmol) was dissolved in dichloromethane (10ml), 4-fluoropiperidine hydrochloride (3A) (0.155g, 1.50mmol) (prepared according to US20110207704) and triethylamine (0.121g) were added, After reacting at room temperature for 1 hour, acetic acid (0.132 g, 2.20 mmol) was added. After stirring at room temperature for 3 hours, sodium triacetoxyborohydride (0.636 g, 3.00 mmol) was added, and the reaction was terminated after stirring overnight at room temperature. Concentrate under reduced pressure to remove most of the reaction solvent, slowly add saturated aqueous sodium bicarbonate solution (50 mL) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com