Method for synthesizing quinoxaline compounds by double-protein catalytic cascade reaction
A cascade reaction and compound technology, applied in the field of biocatalytic synthesis, can solve the problems of too many synthesis steps and insufficient environmental protection, and achieve the effects of simple process, reduced separation and purification steps, and reduced use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A method for synthesizing quinoxaline compounds through double protein catalyzed cascade reaction, the steps are as follows:
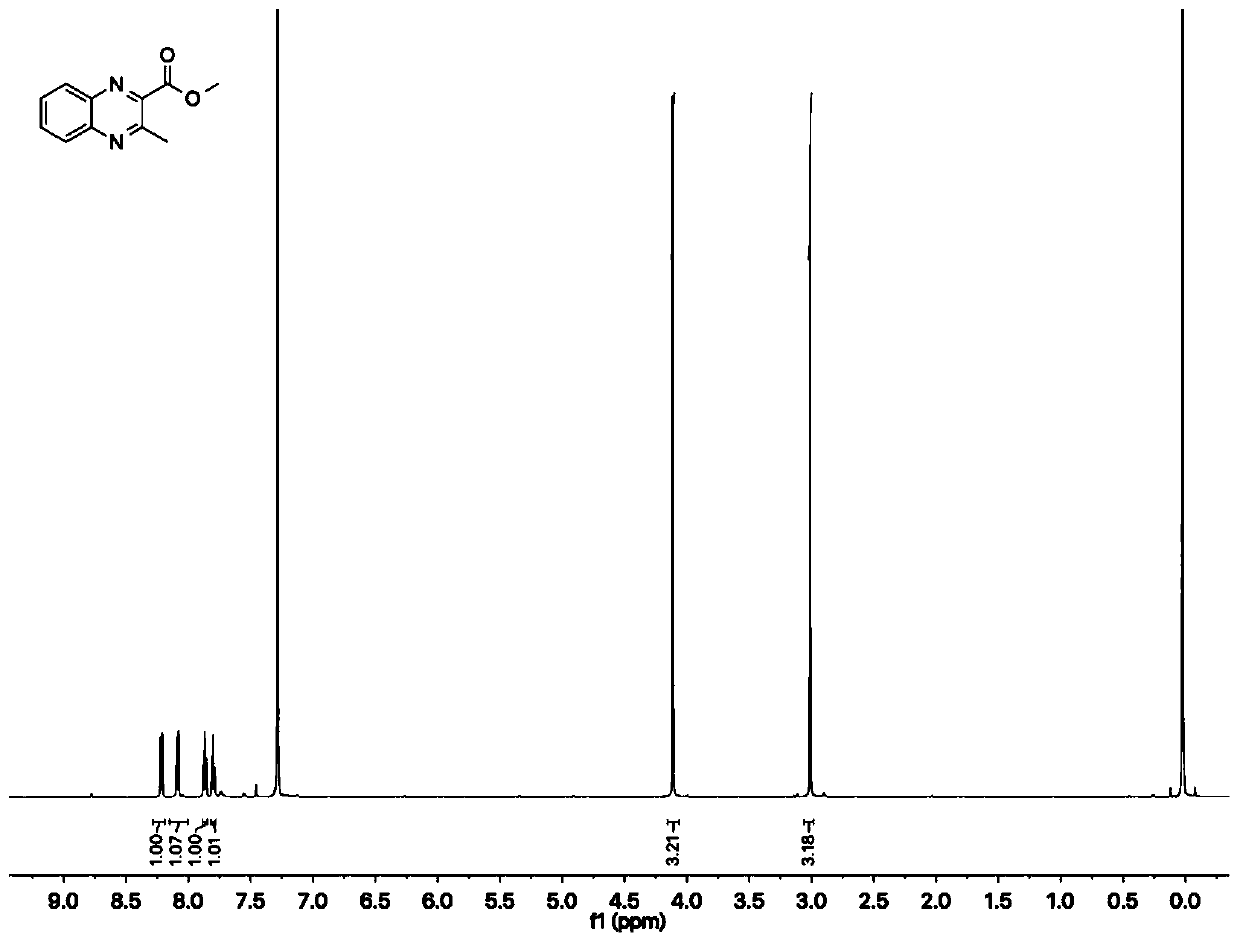
[0046] In 2ml of water, bovine hemoglobin (0.1mol%, i.e. 1 μmol) and porcine chymolipase (PPL, 20mg) were used as catalysts, and after adding 5%mol of TritonX-100 as a surfactant, 108.0mg of phthalate was added Diamine, 139.2 mg of methyl acetoacetate and 197.2 mg of p-toluenesulfonyl azide were stirred and reacted at 55° C. for 6 h. The reaction progress was detected by thin-layer chromatography. After the reaction was completed, ethyl acetate was added for extraction, and then washed with hydrochloric acid, dried with anhydrous magnesium sulfate, and concentrated. Further purify with silica gel column chromatography (ethyl acetate / n-hexane) afterwards, obtain quinoxaline compound (methyl 3-methyl-2-quinoxaline carboxylate 183.82mg, white solid, productive rate 91% ).
[0047] Concrete reaction formula is as follows:
[0048]
[0049] Fur...
Embodiment 2
[0053] A method for synthesizing quinoxaline compounds through double protein catalyzed cascade reaction, the steps are as follows:
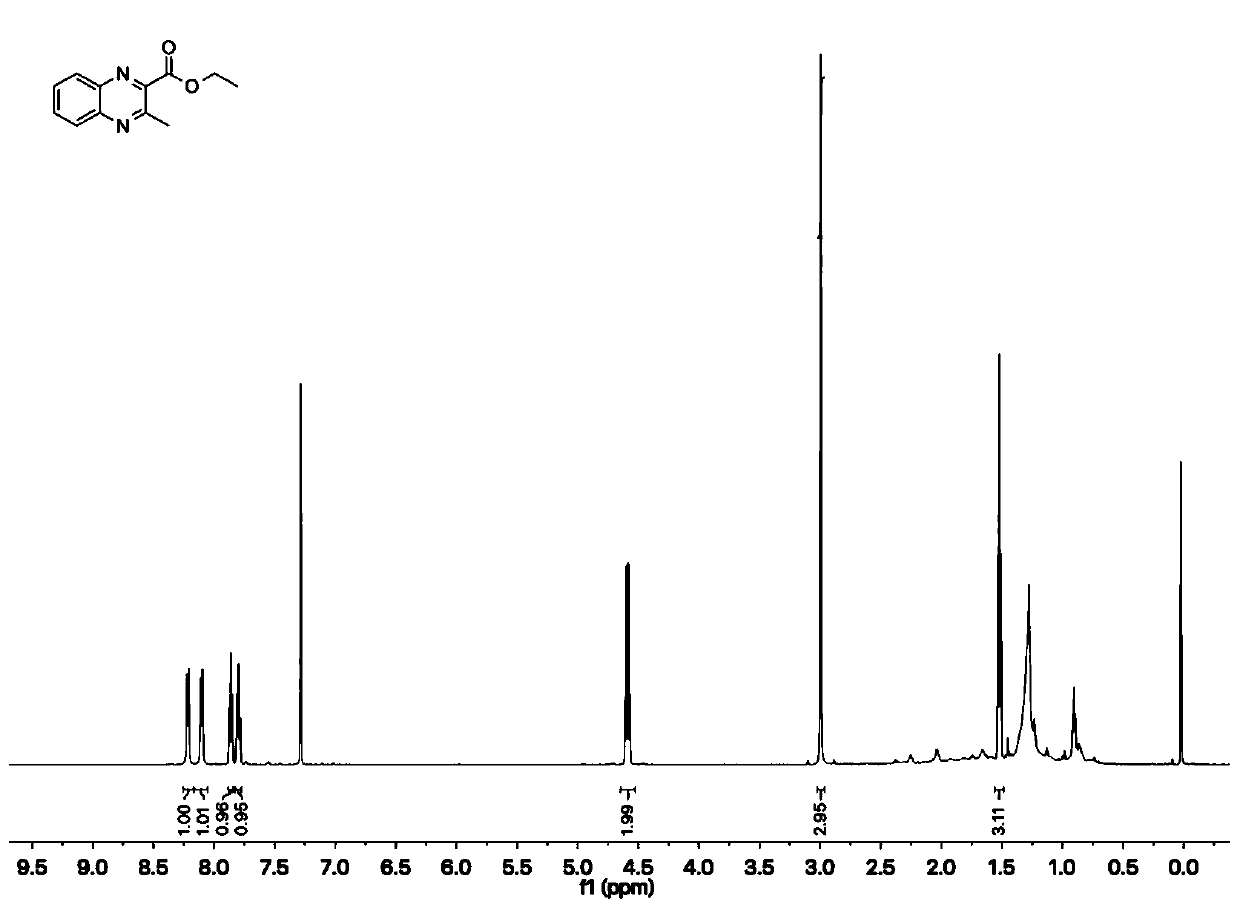
[0054] In 2ml of water, bovine hemoglobin (0.1mol%, i.e. 1 μmol) and porcine chymolipase (PPL, 20mg) were used as catalysts, and after adding 5%mol of TritonX-100 as a surfactant, 108.0mg of phthalate was added Diamine, 156.0 mg of ethyl acetoacetate and 197.2 mg of p-toluenesulfonyl azide were stirred and reacted at 55° C. for 6 h. The reaction progress was detected by thin-layer chromatography. After the reaction was completed, ethyl acetate was added for extraction, and then washed with hydrochloric acid, dried with anhydrous magnesium sulfate, and concentrated. Afterwards, it was further purified by silica gel column chromatography (ethyl acetate / n-hexane) to obtain quinoxaline compounds (ethyl 3-methyl-2-quinoxaline carboxylate 194.6 mg; yield 90%, white solid) .
[0055] Concrete reaction formula is as follows:
[0056]
[0057] Furt...
Embodiment 3
[0061] A method for synthesizing quinoxaline compounds through double protein catalyzed cascade reaction, the steps are as follows:
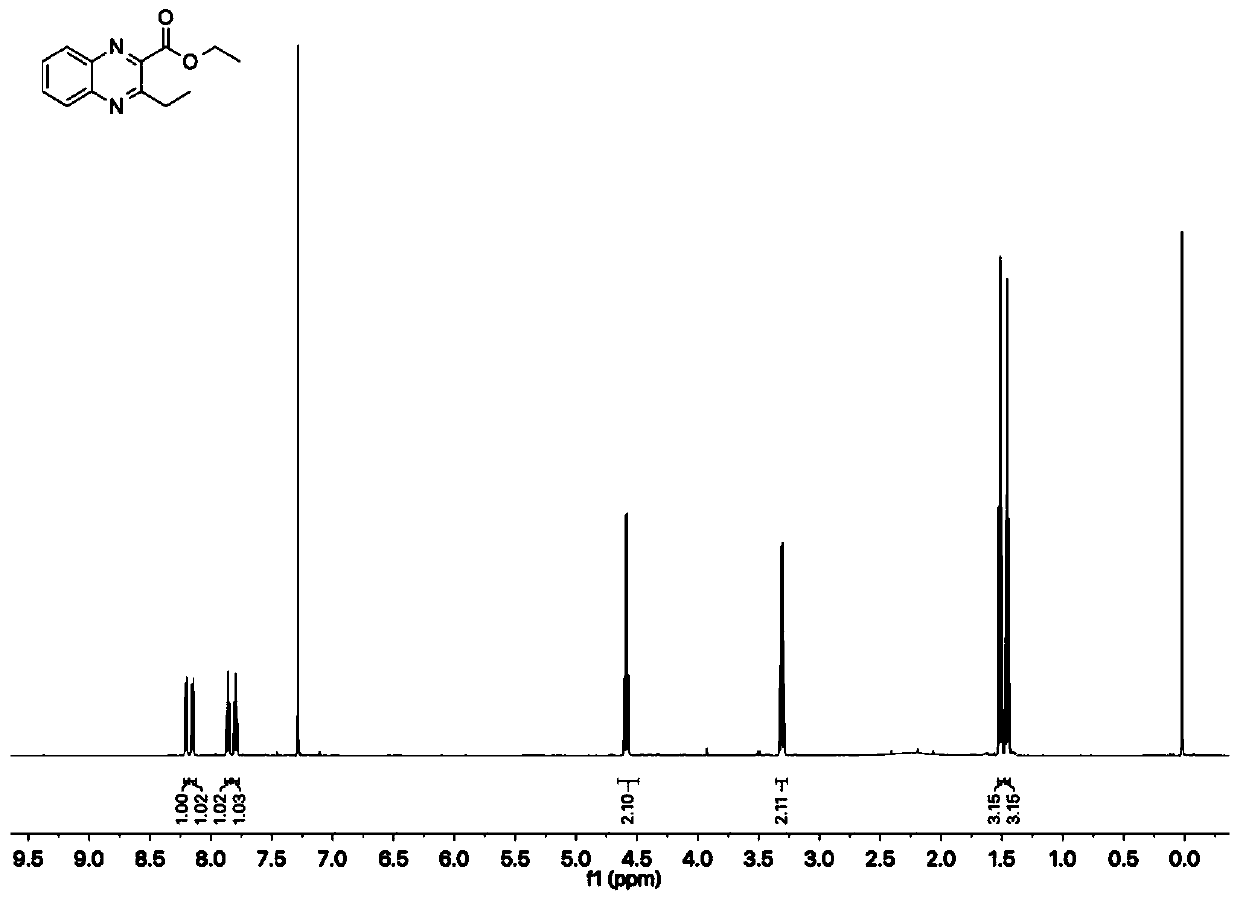
[0062] In 2ml of water, bovine hemoglobin (0.1mol%, i.e. 1 μmol) and porcine chymolipase (PPL, 20mg) were used as catalysts, and after adding 5%mol of TritonX-100 as a surfactant, 108.0mg of phthalate was added Diamine, 172.8mg of ethyl propionoacetate and 197.2mg of p-toluenesulfonyl azide were stirred and reacted at 55°C for 6h. The reaction progress was detected by thin-layer chromatography. After the reaction was completed, ethyl acetate was added for extraction, and then washed with hydrochloric acid, dried with anhydrous magnesium sulfate, and concentrated. Afterwards, it was further purified by silica gel column chromatography (ethyl acetate / n-hexane) to obtain quinoxaline compounds (ethyl 3-ethyl-2-quinoxaline carboxylate 202.6 mg; yield 88%, white solid) .
[0063] Concrete reaction formula is as follows:
[0064]
[0065] Further...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com