A kind of amino acid dehydrogenase and its application
A technology of amino acid dehydrogenase and amino acid, applied in the direction of enzymes, oxidoreductases, biochemical equipment and methods, etc., can solve the problems of complex reaction process, serious environmental pollution, harsh reaction conditions, etc., and achieve simple equipment and high optical purity , the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] To construct an amino acid dehydrogenase mutant with a single point mutation (G60S), and to mutate the 60th amino acid, the specific steps are as follows:
[0029] (1) Introduce mutations: design primers according to the nucleotides shown in SEQ ID NO.02, and design forward and reverse primers containing different sites. The forward and reverse primers are as follows:
[0030] Upstream primer (SEQ ID NO.05): G60S-F GTGCTGAGATTGTCAAAATCAATG
[0031] Downstream primer (SEQ ID NO.06): G60S-R TGATTTTGACAATCTCAGCACATC
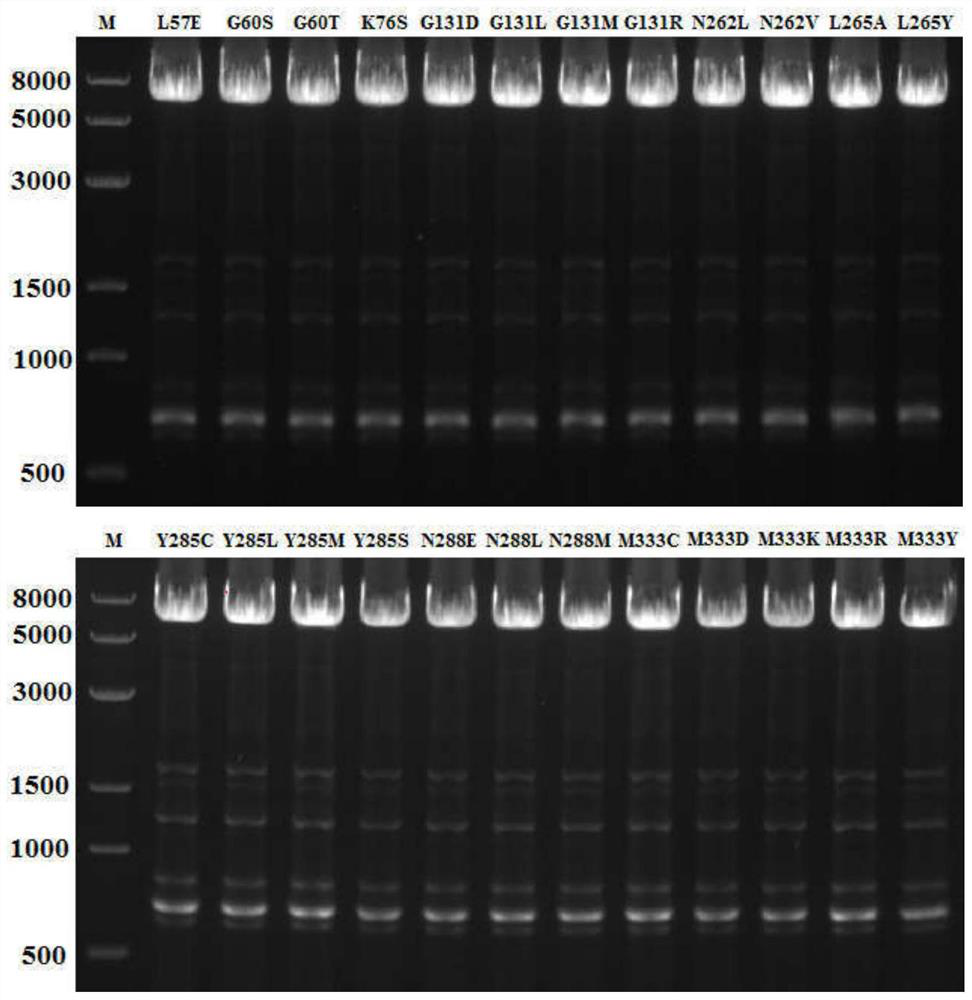
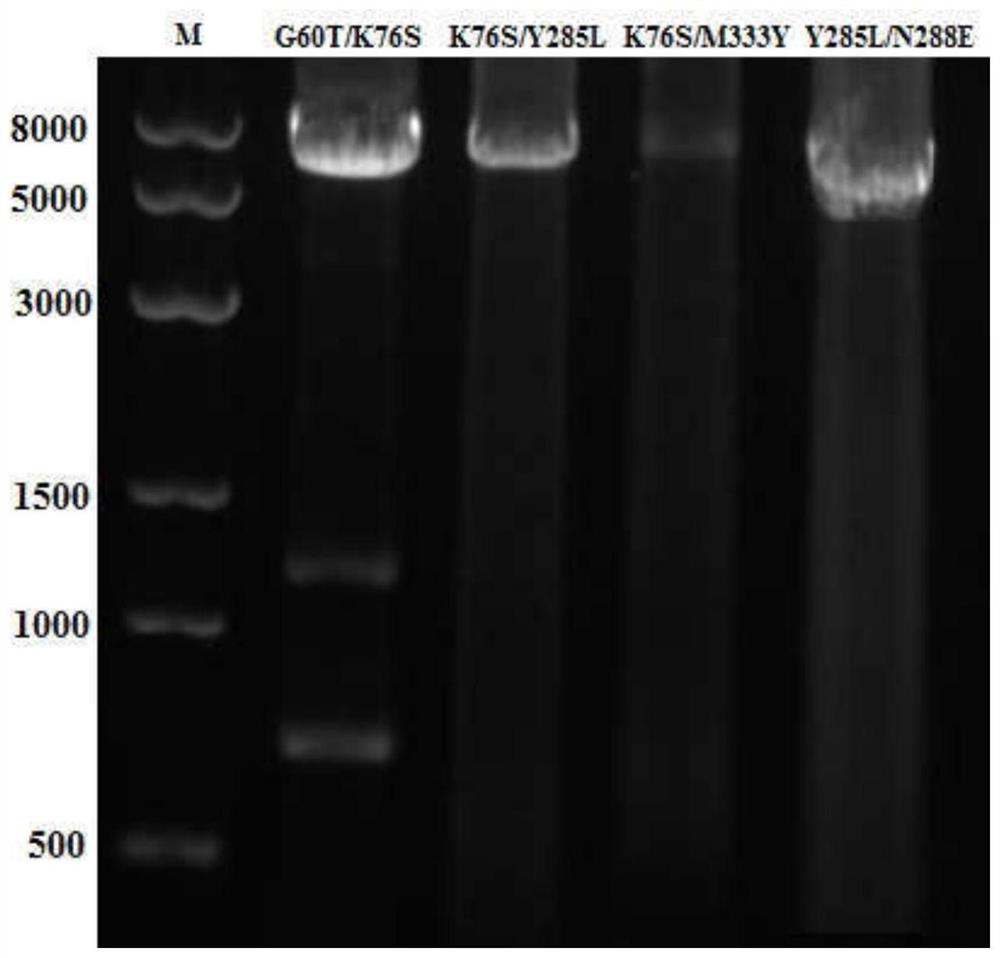
[0032] After mixing the primers and the template plasmid, add high-fidelity Taq polymerase KOD-Plus to carry out PCR amplification of the whole plasmid, and electrophoresis to detect the PCR product after PCR, as figure 1 shown.
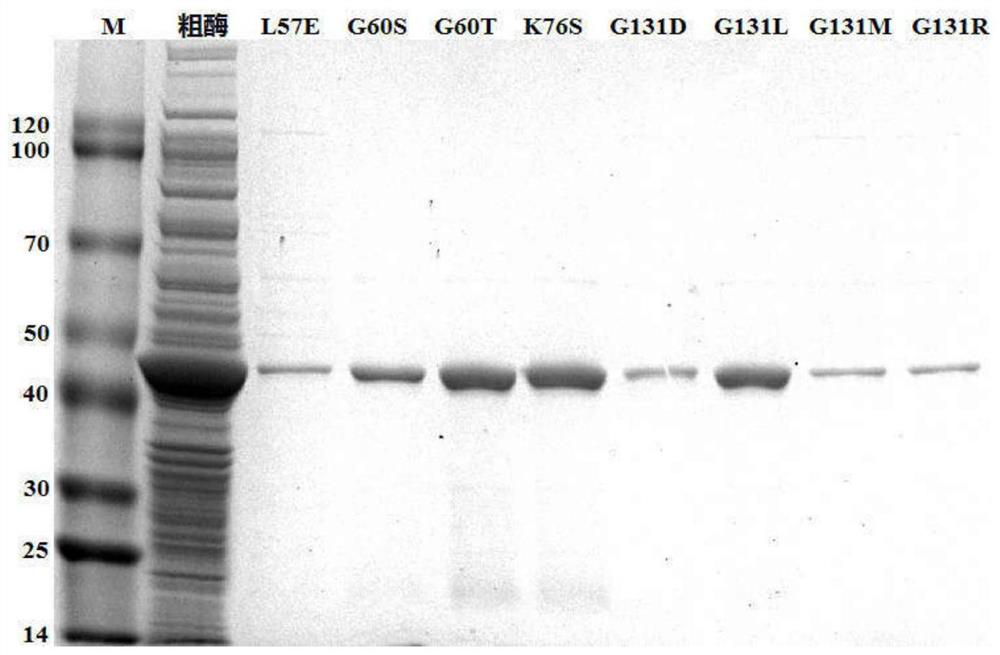
[0033] (2) Preparation of crude enzyme solution: construct a recombinant expression strain capable of expressing phenylalanine dehydrogenase with a His-tag tag: the original sequence of the phenylalanine dehydrogenase (PheDH) gene...
Embodiment 2
[0039] Construct an amino acid dehydrogenase mutant with a single point mutation (K76S), and mutate the 76th amino acid. The specific steps are as follows:
[0040] (1) Introduce mutations: design primers according to the nucleotides shown in SEQ ID NO.02, and design forward and reverse primers containing different sites. The forward and reverse primers are as follows:
[0041] Upstream primer (SEQ ID NO.9): K76S-F CTTCGGTGGAGGTTCATCGGTCAT
[0042] Downstream primer (SEQ ID NO.10): K76S-R GAACCTCCACCGAAGTCAACATCT
[0043] After mixing the primers and the template plasmid, add high-fidelity Taq polymerase KOD-Plus to carry out PCR amplification of the whole plasmid, and electrophoresis to detect the PCR product after PCR, as figure 1 shown.
[0044] Experimental steps (2)-(3) are the same as steps (2)-(3) in Example 1.
[0045] (4) Enzyme activity detection: the catalytic reaction system contains 100mM NADH, 0.4mol / L glycine-sodium hydroxide buffer solution (pH 10.5), co-sol...
Embodiment 3
[0048] Construct an amino acid dehydrogenase mutant with a single point mutation (M333D), and mutate the 333-position amino acid. The specific steps are as follows:
[0049] (1) Introduce mutations: design primers according to the nucleotides shown in SEQ ID NO.02, and design forward and reverse primers containing different sites. The forward and reverse primers are as follows:
[0050] Upstream primer (SEQ ID NO.17): M333D-FCAGATATCTACGGATGAAGCAGCA
[0051] Downstream primer (SEQ ID NO.18): M333D-R ATCCGTAGATATCTGGTTCTTTGT
[0052] After mixing the primers and the template plasmid, high-fidelity Taq polymerase KOD-Plus was added to perform PCR amplification of the whole plasmid, and the PCR product was detected by electrophoresis after PCR.
[0053] Experimental steps (2)-(3) are the same as steps (2)-(3) in Example 1.
[0054] (4) Enzyme activity detection: the catalytic reaction system contains 20mM NADH, 0.2mol / L glycine-sodium hydroxide buffer solution (pH 8.0), and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com