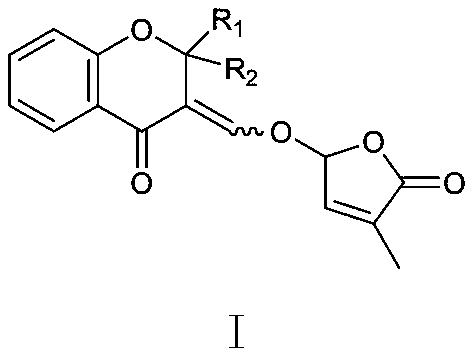
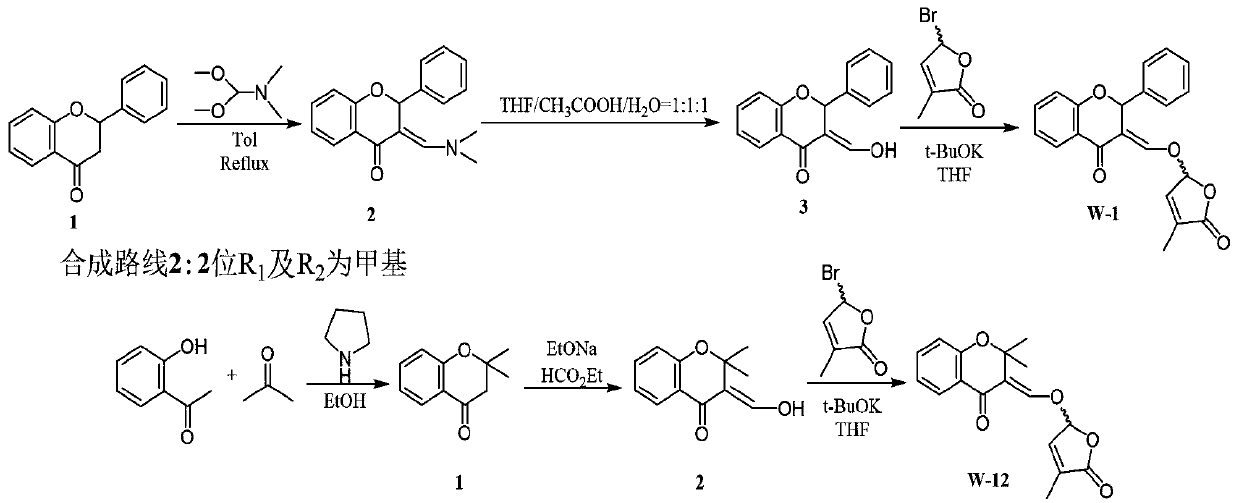
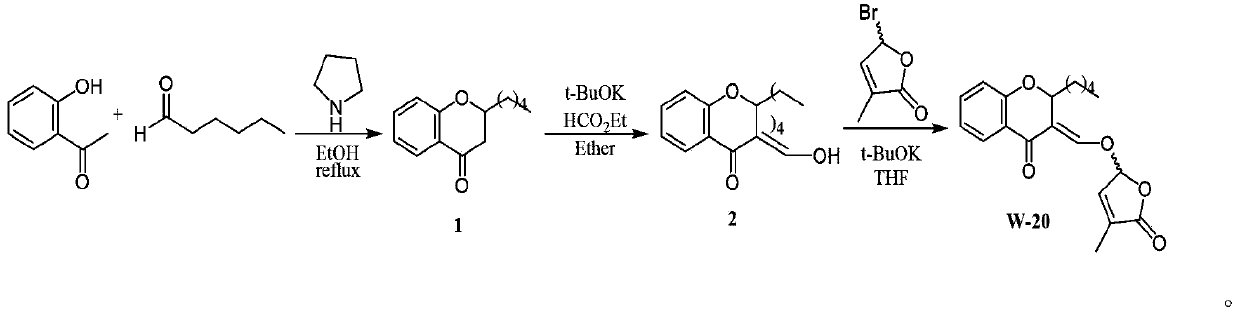
Synthesis and application of lactone analogue with flavone framework
A technology of analogs and skeletons, applied in the field of Striga, can solve the problems of complex structure, difficult to synthesize in large quantities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthetic route 1:
[0022] 1.36ml of o-hydroxyacetophenone (1.2eq), 0.96ml of benzaldehyde (1eq), 1.29ml of aniline (1.5eq) were dissolved in 15ml of methanol, then 0.72g of iodine was added and the reaction was stirred at 40°C for 20 hours. The reaction process was monitored by TLC. After the substrate was completely consumed, the reaction system was concentrated and directly separated by silica gel column chromatography (petroleum ether: ethyl acetate = 200:1) to obtain 1.3 g of white solid 1 with a yield of 61.5%.
[0023] Take 0.68g of compound 1 (1eq) and dissolve it in 10ml of toluene, then add 1.21ml of N,N-dimethylformamide dimethyl acetal (3eq) to the reaction system, reflux at 150°C for 6-8 hours and TLC monitoring, after the reaction was completed, it was directly separated by silica gel column chromatography (petroleum ether: ethyl acetate = 2:1) to obtain 0.68 g of light yellow solid 2 with a yield of 80.4%.
[0024] Dissolve 0.68g of compound 2 (1eq) in ...
Embodiment 2
[0045] Compounds W-1~W-24 were tested for the germination activity of melon radang and sunflower radang seeds, and the test method was as follows:
[0046] Take a plastic Petri dish with a diameter of 9 cm, lay a piece of filter paper on the bottom layer and moisten it with sterilized distilled water, and then cover it with a filter paper sheet with a diameter of 6 mm. Sprinkle the melon seeds evenly on the wet filter paper, and the number of seeds on the filter paper is about 25-65. Seal the Petri dish with sealant and pre-incubate the seeds for 3-7 days at room temperature in the dark. Take a glass fiber filter paper sheet with a diameter of 6 mm, place it in a plastic petri dish and add 25 μL of the compound solution to be tested (acetone as a solvent), after the acetone is completely volatilized, take a pre-cultured seed piece and place it on top of it, and add 25 μL of sterilized distilled water , and finally place a piece of filter paper moistened with sterilized distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com