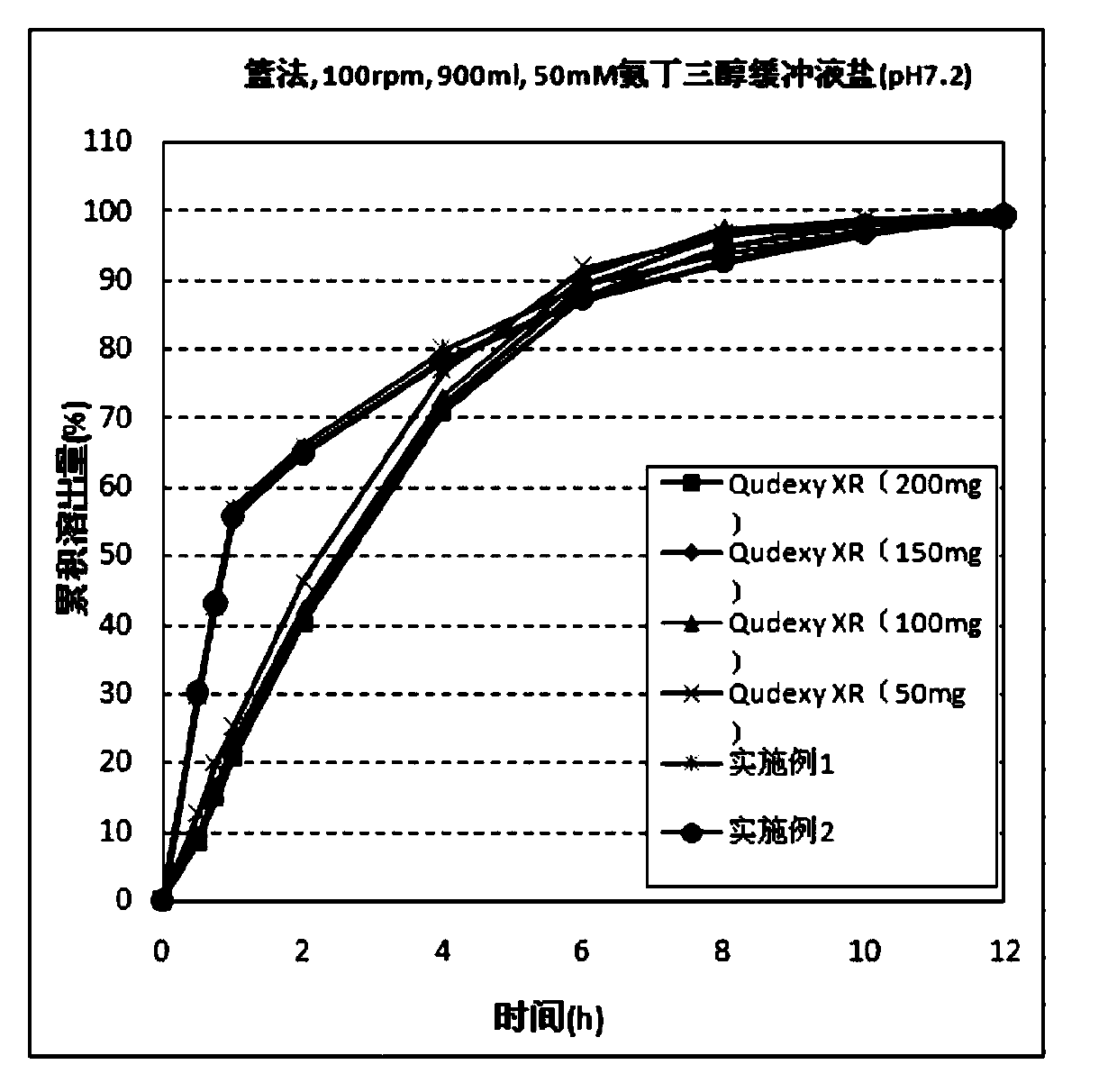
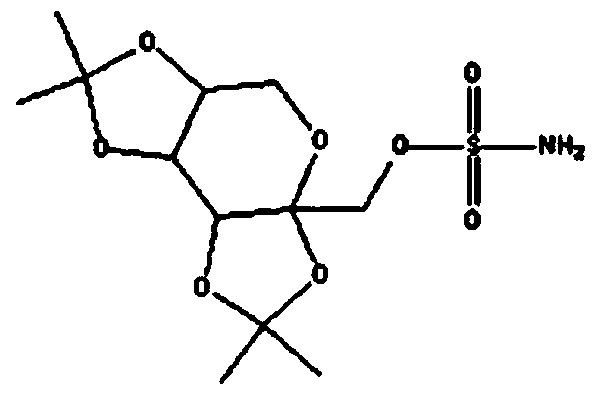
Topiramate sustained-release capsule and preparation method thereof
A technology for sustained-release capsules and topiramate, which is applied in directions such as capsule delivery, microcapsules, and pharmaceutical formulations, can solve problems such as complex preparation processes and achieve the effect of stabilizing blood drug concentrations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
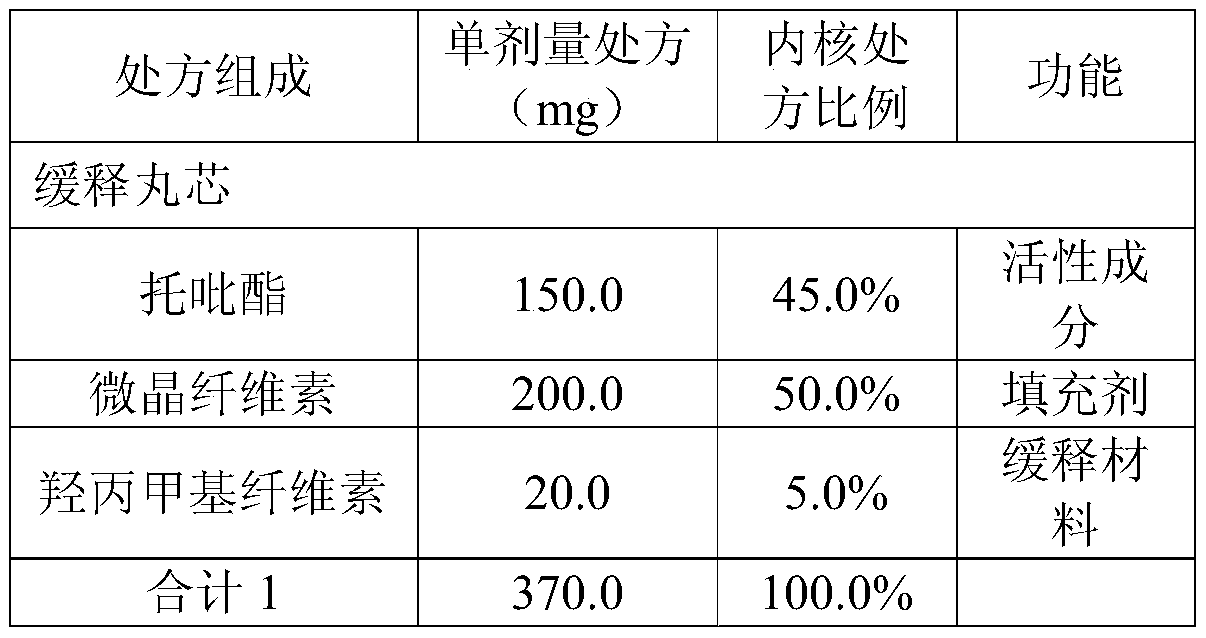
[0027] Embodiment 1: preparation of topiramate sustained-release capsules
[0028] prescription:
[0029]
[0030]
[0031] Preparation Process:
[0032] (1) Preparation of drug-containing sustained-release pellets by extrusion spheronization technology
[0033] Topiramate, microcrystalline cellulose (filler) and hypromellose (binder) of recipe quantity are mixed homogeneously in Mycromix high-shear wet granulator, and process parameter is as follows:
[0034]
[0035] After premixing, add purified water to the premix powder for granulation. The process parameters are as follows:
[0036]
[0037] After the wet granulation is completed, the material is discharged, and the prepared soft material is pelletized through the E550 / S-250 extrusion spheronizer. The process parameters are as follows:
[0038]
[0039] After pellet making, use a fluidized bed to dry the wet pellets until the water content is less than 5%, to obtain drug-loaded sustained-release pellets...
Embodiment 2
[0051] Embodiment 2: Preparation of topiramate sustained-release capsules
[0052] prescription:
[0053]
[0054]
[0055] Preparation Process:
[0056] (1) Preparation of drug-loaded immediate-release pellets
[0057] Dissolve the prescribed amount of hydroxypropyl methylcellulose in purified water, then add the prescribed amount of topiramate, homogenize for 5-10 minutes with a high-shear emulsifier, prepare a suspension coating solution, and coat the coating solution containing topiramate On the outside of the blank microcrystalline pellet core, the immediate-release pellets were obtained, and the parameters of the coating process were as follows:
[0058]
[0059] (2) Sustained release layer coating
[0060] Dissolve the prescribed amount of ethyl cellulose and PEG-6000 in ethanol solution, then add magnesium stearate, homogenize for 5-10 minutes in a high-shear emulsifier, prepare a slow-release layer coating solution, and use a fluidized bed to The coating ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com