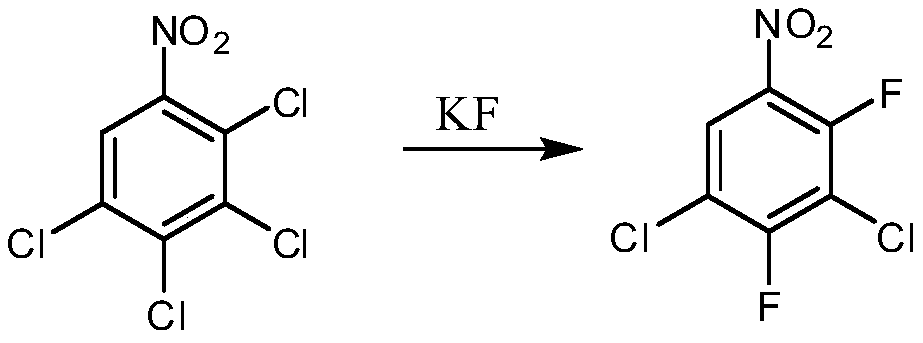
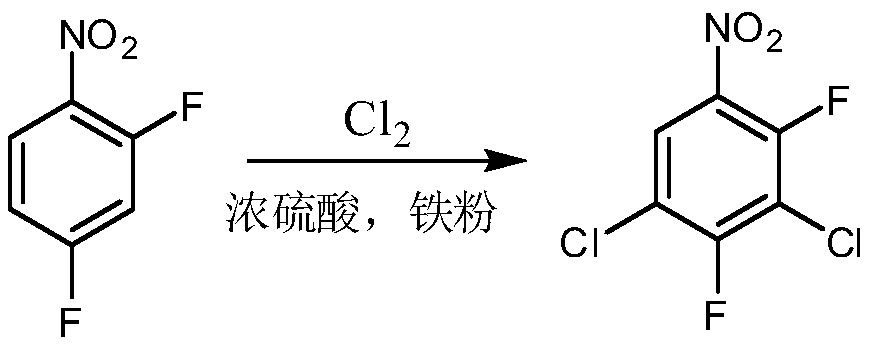
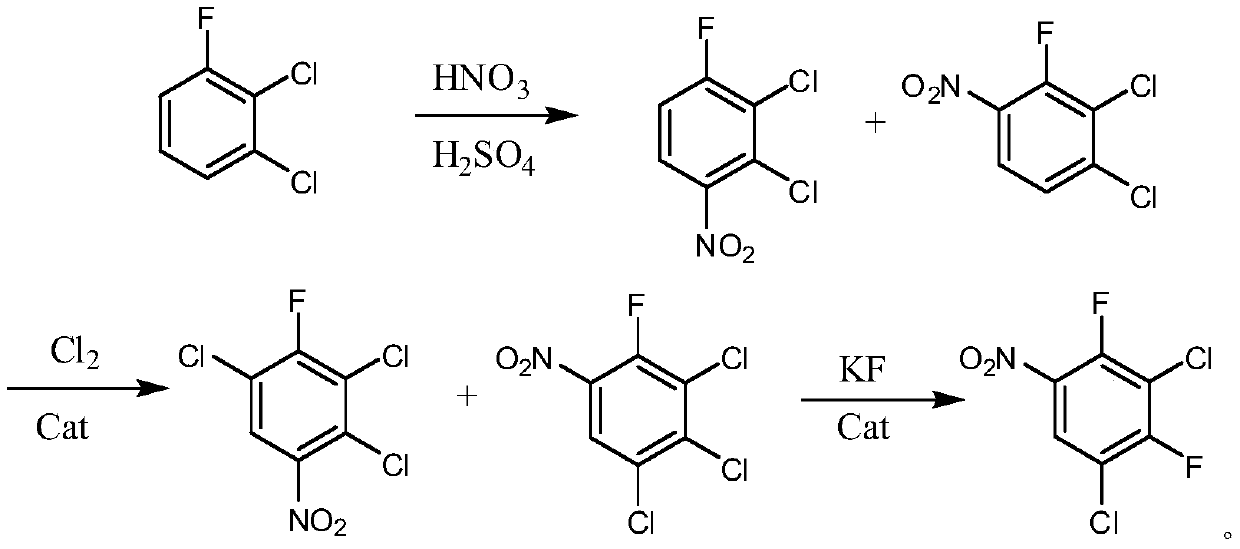
Preparation method of 2,4-difluoro-3,5-dichloronitrobenzene
A technology of dichloronitrobenzene and fluoronitrobenzene, which is applied in the field of preparation of pesticide intermediates, can solve the problems of poor selectivity, slow reaction, and difficulty in processing, and achieve good conversion rate and yield, accelerated reaction speed, and excellent reaction conditions simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Add 165g of 2,3-dichlorofluorobenzene to a 500mL four-neck flask, use 70.7g of fuming nitric acid (98%) and 70.7g of concentrated sulfuric acid (98%) to make a mixed acid, and add the mixed acid dropwise at 50-60°C, After dripping, keep it at the same temperature for 3 hours, let it stand for half an hour, separate the layers, wash the organic layer with water and alkali respectively, and obtain 206.9 g of the nitration mixture by layering. 2,3-dichloro-4-fluoronitrobenzene 86.2%, 2-fluoro-3,4-dichloronitrobenzene 13.5%, molar yield 98.5%. GC: gas chromatography.
[0037] (2) Add 200g of the nitration mixture to a 500mL four-neck flask, add 10g of ferric chloride and 2g of sodium iodide, raise the temperature to 60-70°C, inject chlorine gas, and react for about 30 hours. When 2,3-dichloro-4-fluoro When nitrobenzene is less than 2%, the reaction is terminated (at this time, 2-fluoro-3,4-dichloronitrobenzene has been completely converted), and the reaction solution i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com