Drug composition containing rosuvastatin calcium and preparation method and application thereof
A technology of rosuvastatin calcium and its composition, which is applied in the direction of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of no type I allergy curative effect, and achieve excellent curative effect and reasonable prescription , the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] This embodiment also provides a method for preparing the above-mentioned first composition and second composition, which includes:
[0025] Step S1: In parts by weight, microcrystalline cellulose, lactose, tricalcium phosphate and part of cross-linked povidone are mixed, granulated by wet method, and granulated with a 20-40 mesh sieve after drying to obtain blank granules;
[0026] Further, it also includes the step of uniformly mixing 1800-4200 parts by weight of seaweed polysaccharide, lactose, microcrystalline cellulose, tricalcium phosphate and partially cross-linked povidone, and performing wet granulation.
[0027] Furthermore, the drying temperature after granulation by the manufacturing method is 55-65 degreeC.
[0028] Step S2: mixing the blank granules with rosuvastatin calcium, the remaining crospovidone and magnesium stearate to prepare the preparation.
[0029] Further, the raw material of rosuvastatin calcium is passed through an 80-mesh sieve; the lactos...
Embodiment 1
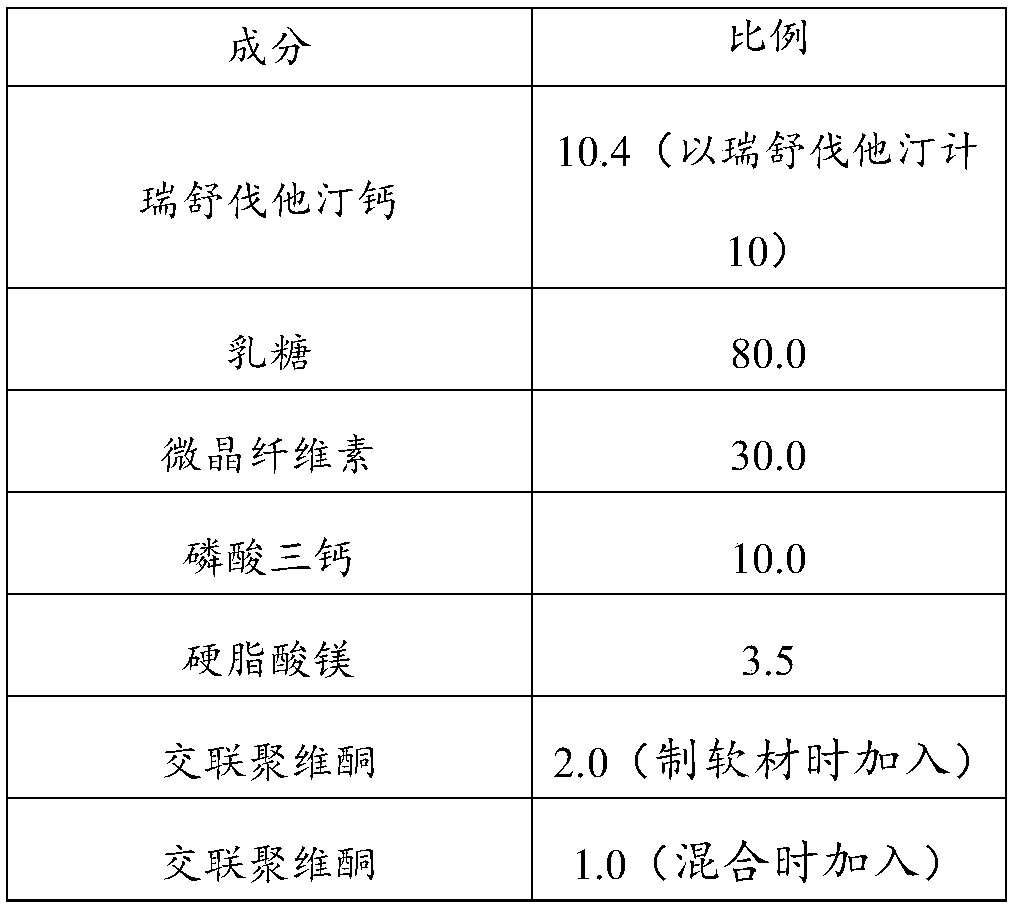
[0036] The present embodiment provides a kind of pharmaceutical composition, and its composition and dosage are as shown in the following table:
[0037]
[0038] The preparation method of this pharmaceutical composition comprises:
[0039] a. Making soft materials and granulating: take by weighing microcrystalline cellulose, lactose (200 mesh), tricalcium phosphate, and cross-linked povidone, mix evenly, add an appropriate amount of wetting agent (ethanol-water mixed solution) to prepare Soft material, 30 mesh sieve nylon granulation;
[0040] b. Drying and sizing: Boiling drying at 60°C, sizing with a 30-mesh nylon sieve to obtain blank granules;
[0041] c. Total blending: according to the amount of blank granules, add the raw material drug and crospovidone in the prescribed proportion, mix evenly, then add the magnesium stearate in the prescribed amount, and mix evenly;
[0042] d. Tablet compression: Use a tablet press machine with a diameter of 7 mm to punch the ble...
Embodiment 2
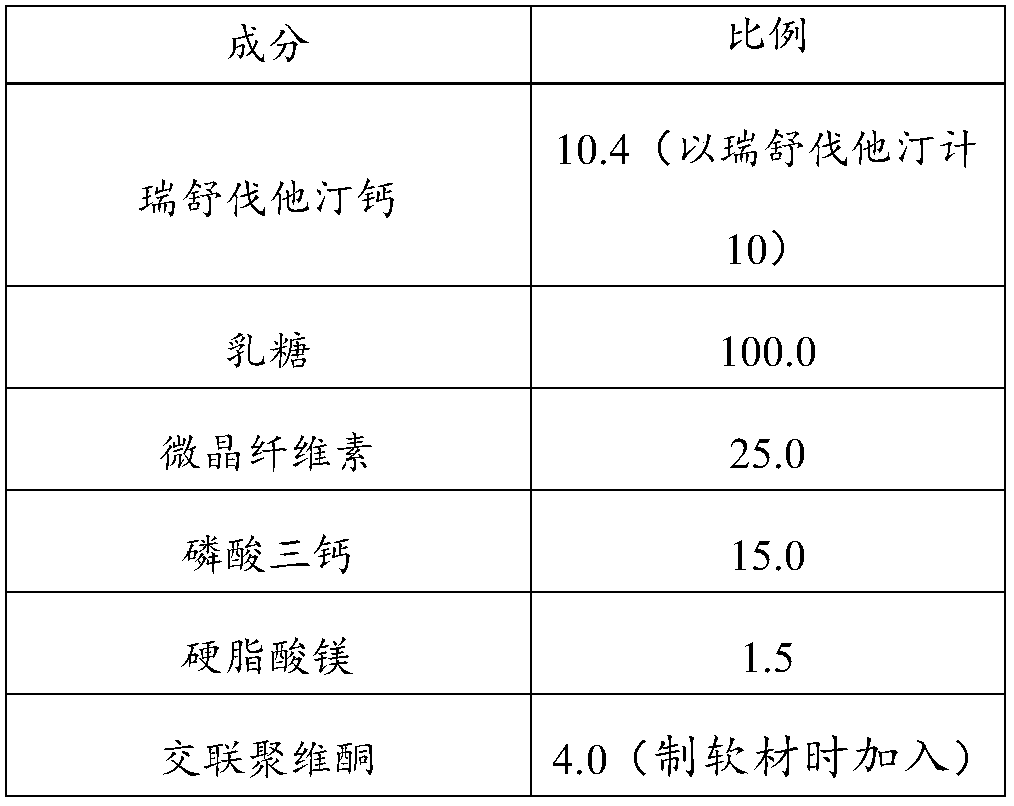
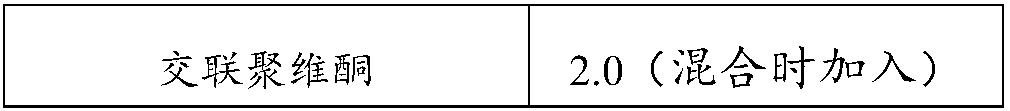
[0045] The present embodiment provides a kind of pharmaceutical composition, and its composition and dosage are as shown in the following table:
[0046]
[0047]
[0048] The preparation method of this pharmaceutical composition comprises:
[0049] a. Making soft materials and granulating: take by weighing microcrystalline cellulose, lactose (200 mesh), tricalcium phosphate, and cross-linked povidone, mix evenly, add an appropriate amount of wetting agent (ethanol-water mixed solution) to prepare Soft material, 20 mesh sieve nylon granulation;
[0050] b. Drying and sizing: Boiling drying at 55°C, sizing with a 20-mesh nylon sieve to obtain blank granules;
[0051] c. Total blending: according to the amount of blank granules, add the raw material drug and crospovidone in the prescribed proportion, mix evenly, then add the magnesium stearate in the prescribed amount, and mix evenly;
[0052] d. Tablet compression: use a tablet press machine to punch the blended granule...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com