Didemethylated actinomycin derivative and application thereof in preparation of drugs for resisting drug-resistant bacteria infection
A technology of actinomycin and double demethylation, applied in the field of natural products, can solve problems such as high toxicity and restricted use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
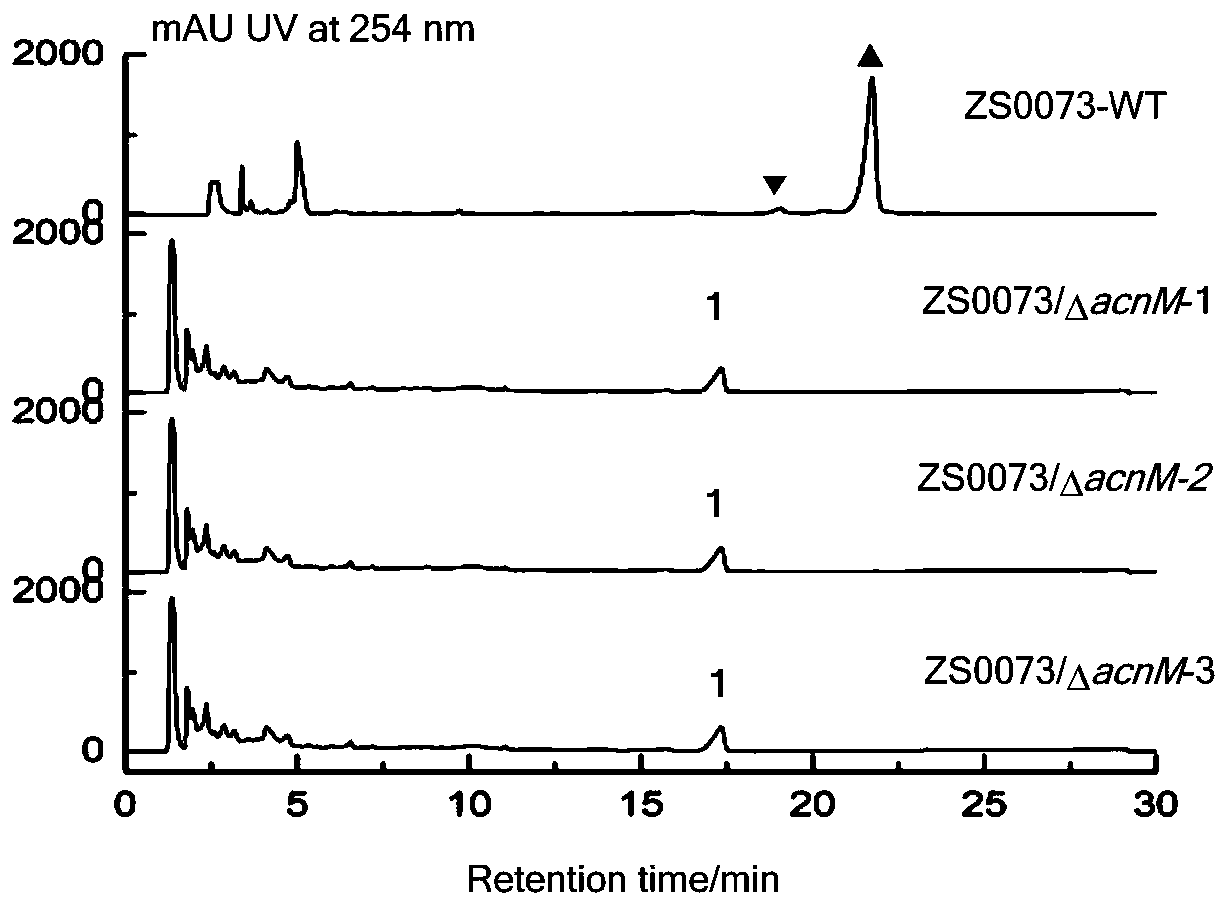
[0023] Example 1: Knockout of methyltransferase genes in the actinomycin D biosynthetic gene cluster of marine-derived Streptomyces S. costaricanus SCSIO ZS0073
[0024] The Streptomyces S. costaricanus SCSIO ZS0073 involved in the present invention is isolated from the soil of the South China Sea mangrove forest. The methyltransferase in the gene cluster was knocked out by insertional inactivation to construct the mutant strain ZS0073 / ΔacnM. ZS0073 / ΔacnM strains grown on ISP2 medium were inoculated into 250mL Erlenmeyer flasks containing 50mL ISP2 medium, and fermented and cultured at 28°C and 200rpm for seven days. The fermented medium was extracted with butanone and analyzed by HPLC. It was found that ZS0073 / ΔacnM could produce Didemactinomycins ( figure 1 ).
[0025] Among them, the composition of ISP2 medium is as follows: 4.0g / L glucose, 4.0g / L yeast extract powder, 10g / L malt extract powder, 30g / L sea salt, 20g / L agar powder, the solvent is water, and the pH value is ...
Embodiment 2D
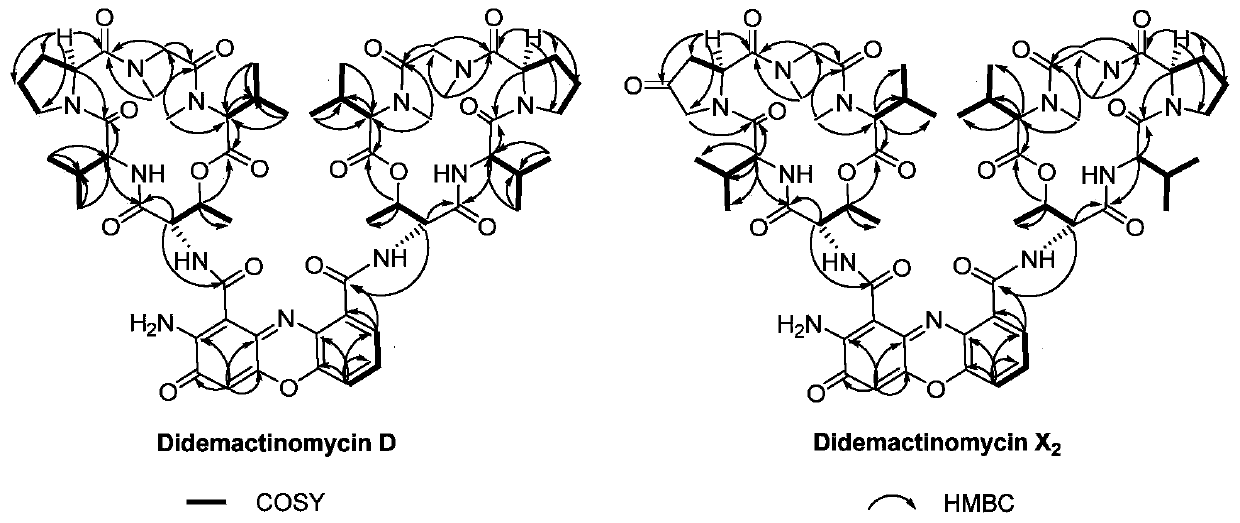
[0026] Isolation and structural identification of embodiment 2Didemactinomycins
[0027] 1. Preparation of fermentation culture of marine Streptomyces S. costaricanus SCSIO ZS0073 methyltransferase inactivated mutant strain ZS0073 / ΔacnM
[0028] (1) Preparation of seed medium and fermentation medium:
[0029] Both the seed medium and the fermentation medium are liquid ISP2 medium containing sea salt, the composition of which is as follows: 4.0g / L glucose, 4.0g / L yeast extract powder, 10g / L malt extract powder, 30g / L sea salt, 20g / L Agar powder, the solvent is water, adjust the pH value to 7.3, the preparation method is to mix the ingredients evenly, adjust the pH value, and sterilize; divide the seed culture medium into 250mL conical flasks, 50mL per bottle, 115°C Sterilize for 30min and set aside. The amplified fermentation medium was divided into 1L Erlenmeyer flasks, 200mL per bottle, and sterilized at 115°C for 30min for later use.
[0030] (2) Cultivation of seeds:
[0...
Embodiment 3
[0049] Example 3 Compounds Didemactinomycin D and Didemactinomycin X 2 Test and analysis of antibacterial activity against a series of Staphylococcus aureus
[0050] Testing Compounds Didemactinomycin D and Didemactinomycin X by Microwell Method 2 Inhibitory activity against a series of Staphylococcus aureus. A series of Staphylococcus aureus was cultured in Mueller-Hinton (MH) broth medium. And prepare the sample solution before the experimental bacteria grow well. Concentrations of samples and positive controls were configured, and ampicillin, kanamycin or vancomycin were selected as positive controls. Both the sample and the sample were configured to 3200μg / mL, and both were dissolved in DMSO. Add 92 μL of sterile MH broth to the first column of the 96-well plate with a row gun, add 50 μL of MH broth to the remaining columns, and add 50 μL of sterile MH broth to the 11th and 12th columns, respectively as positive control and For the negative control, mark it and cover ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com