Recombinant lactococcus lactis and tilapia streptococcus agalactiae disease vaccine
A technology of Lactococcus lactis and Streptococcus lactis, applied in the direction of recombinant DNA technology, bacteria, antibacterial drugs, etc., can solve the problems of chemical drug resistance, drug residue quality and safety issues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
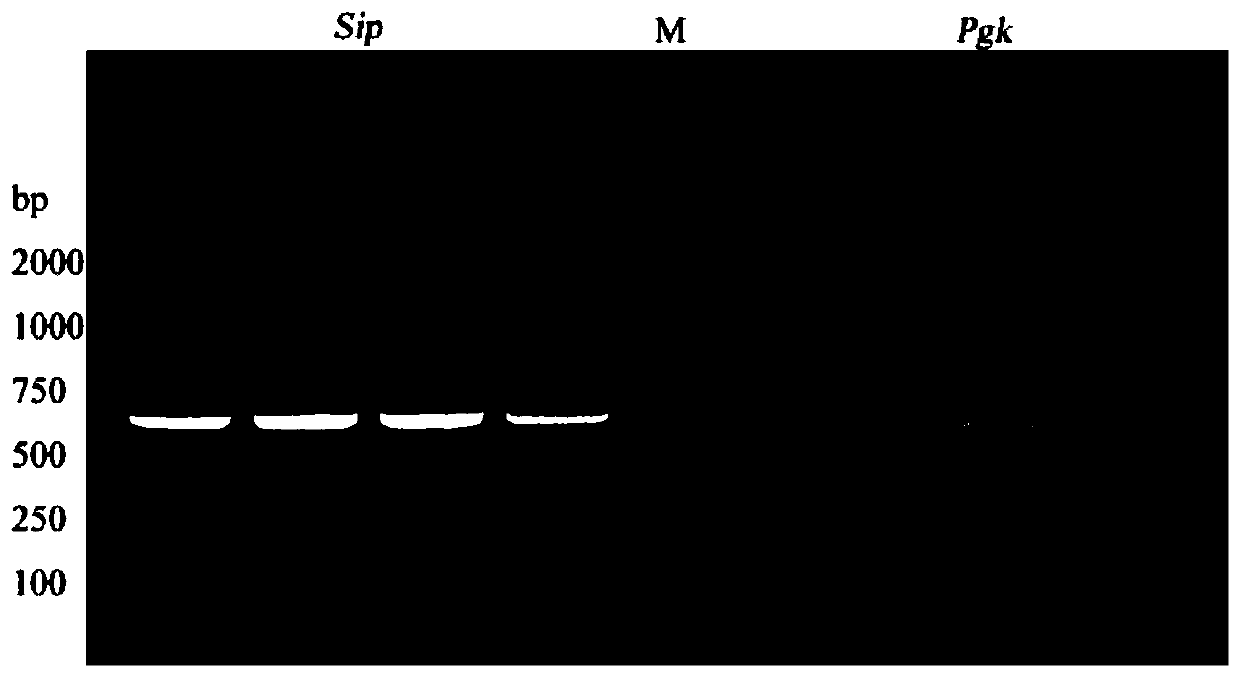
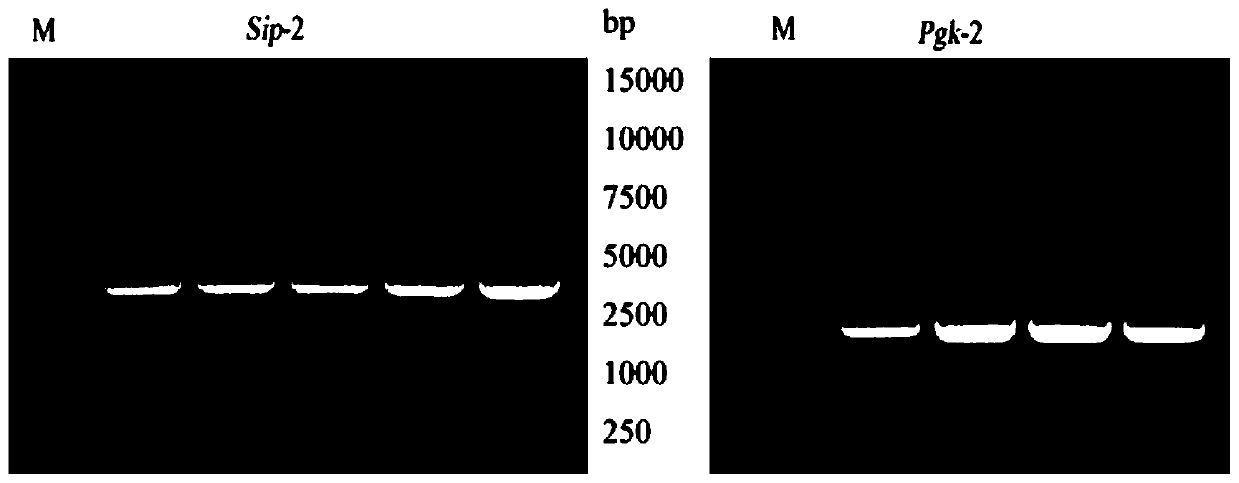
[0025] Embodiment 1: Construction carries the recombinant plasmid of sip-pgk fusion gene
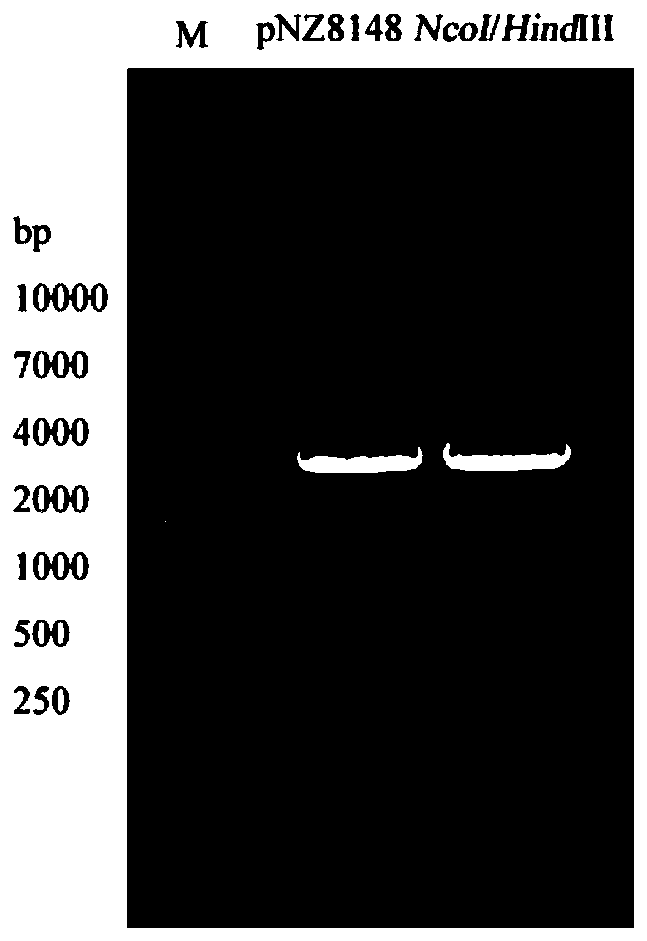
[0026] The sip gene and the pgk gene were amplified from Streptococcus agalactiae (WC1535) preserved by the applicant, and the pNZ8148 plasmid was used as a carrier plasmid to construct a recombinant plasmid carrying the sip-pgk fusion gene.
[0027] The PCR primer sequences for amplifying the sip gene and the pgk gene are shown in Table 1 below, in which a 6×His tag is added to the end of the primer for the target gene, and the general primer of pNZ8148 is selected as the identification primer. The primers used were synthesized at Guangzhou Aiji Biotechnology Co., Ltd. The sequence marked in italics is the 15 bp base sequence homologous to the pNZ8148 vector plasmid, and the sequence marked underlined is the base sequence corresponding to the 6×His tag.
[0028] Table 1
[0029]
[0030] Streptococcus agalactiae WC1535 was cultured in BHI medium (purchased from Guangzhou Kanglong ...
Embodiment 2
[0036] Example 2: Construction of recombinant Lactococcus lactis and identification of fusion gene expression
[0037] First, Lactococcus lactis (Lactococcus. lactis) NZ9000 (L. lactis NZ9000) (purchased from REBIO) competent cells were prepared. A single colony of Lactococcus lactis NZ9000 was cultured statically at 30° C. for 6 h in M17 liquid medium (purchased from Guangzhou Kanglong Biotechnology Co., Ltd.) containing 0.5% glucose. Take the above culture in the M17 liquid medium containing 0.5% glucose + 1% glycine at a ratio of 1:10, and culture overnight at 30°C. According to the ratio of 1:10, take the above culture in the solution containing 0.5% glucose + 0.5mol·L -1 Sucrose + 2% glycine M17 liquid medium, continue static culture to OD 600 = 0.5. Centrifuge the culture at 5000g for 15min at 4°C, discard the supernatant, and add 1 volume of pre-cooled solution (0.5mol·L -1 sucrose+10% glycerin) to resuspend, centrifuge at 5000g at 4°C for 15min, discard the super...
Embodiment 3
[0043] Embodiment 3: the immunoprotective effect of recombinant Lactococcus lactis
[0044] 420 healthy tilapias were selected from Guangdong Tilapia Breeding Farm, with a body length of 8±1cm and a weight of 14±1g.
[0045] These tilapias were randomly divided into 12 groups, 35 fish in each group, and the immune experiment was carried out after the tilapias were kept stable for a while. The 12 groups were divided into 2 large groups, 6 groups received the second immunization 7 days after the first immunization, that is, the second immunization (day 14), and the remaining 6 groups received 7 days after the second immunization (14 days). Carry out the third immunization, that is, three immunizations (day 21), and the immunization method is immunization every other week.
[0046] The six experimental groups were L.lactis NZ9000pNZ8148-sip-pgk (Sip-Pgk fusion protein lactic acid bacteria vaccine), L.lactis NZ9000pNZ8148-sip (Sip protein lactic acid bacteria vaccine control), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com