(S)1-phenyl-1,2,3,4-tetrahydroisoquinoline synthesis method
A technology of tetrahydroisoquinoline and a synthesis method is applied in the field of synthesis of 1-phenyl-1,2,3,4-tetrahydroisoquinoline, and can solve the problems of poor optical purity and low yield of the product, To achieve the effect of simple and safe reaction operation, easy access to raw materials and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
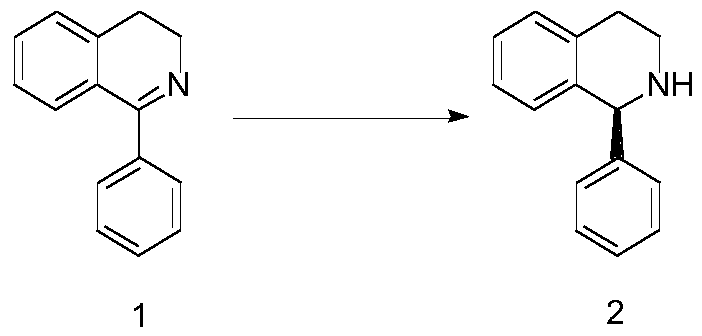
[0018] The embodiment of the present invention provides a synthesis method of (S) 1-phenyl-1,2,3,4-tetrahydroisoquinoline, the reaction route of the synthesis method includes:
[0019]
[0020] Described synthetic method comprises the steps:
[0021] 1) Dissolving raw material 1 in a solvent, adding alkali and catalyst;
[0022] 2) Using hydrogen for gas replacement to form a hydrogen atmosphere, and performing a pressurized reaction to obtain (S)1-phenyl-1,2,3,4-tetrahydroisoquinoline 2;
[0023] Wherein, the catalyst is a BIAMH system catalyst, a D-BIMAH system catalyst or a P-BIMAH system catalyst.
[0024] In the above-mentioned synthetic method, the structural general formula of the BIAMH system catalyst that adopts is:
[0025]
[0026] The general structural formula of the D-BIMAH system catalyst is:
[0027]
[0028] The general structural formula of the P-BIMAH system catalyst is:
[0029] Among them, R 1 For high molecular polymers.
[0030] Preferab...
Embodiment 1
[0043] In this embodiment, a synthetic method of (S)1-phenyl-1,2,3,4-tetrahydroisoquinoline, the steps are as follows:
[0044] 1) In a 5L autoclave, under an argon atmosphere, add 100g of the raw material reactant 1-phenyl-dihydroquinoline (1) through the feeding port, then add 1L of ethanol to fully dissolve the raw material (1), and continue to Inject argon for bubbling degassing, continue bubbling for 1 hour, and degassing is completed;
[0045] 2) Then, under argon atmosphere, add 0.5g catalyst (S)-diopRuCl from the feeding port 2 (R)-P-Me-BIMAH (purchased from Zhongshan Yi Antai Pharmaceutical Technology Co., Ltd.), add 12g of potassium tert-butoxide, after the feeding is completed, quickly close the feeding port; replace the argon with hydrogen, slowly feed the hydrogen to 30atm, Close the inflation valve and stir the reaction at 25-35°C; when the pressure drops to remain constant, the reaction is considered to be stopped, and the sample is sent for liquid phase analys...
Embodiment 2
[0048] In this embodiment, a synthetic method of (S)1-phenyl-1,2,3,4-tetrahydroisoquinoline, the steps are the same as in Example 1, the difference is that the catalyst used is (S)-diopRuCl 2 (R)-Me-BIMAH (BIAMH system catalyst).
[0049] Specific steps are as follows:
[0050] 1) In a 5L autoclave, under an argon atmosphere, add 100g of the raw material reactant 1-phenyl-dihydroquinoline (1) through the feeding port, then add 1L of ethanol to fully dissolve the raw material (1), and continue to Inject argon for bubbling degassing, continue bubbling for 1 hour, and degassing is completed;
[0051] 2) Then, under argon atmosphere, add 0.5g catalyst (S)-diopRuCl from the feeding port 2 (R)-Me-BIMAH (purchased from Zhongshan Yiantai Pharmaceutical Technology Co., Ltd.), finally add 12g of potassium tert-butoxide, after the addition, quickly close the feeding port; replace the argon with hydrogen, slowly introduce hydrogen to 30atm, close Inflate the valve and stir the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com