Composition and immune cells for enhancing T lymphocyte immunity and application
A technology of lymphocytes and immune cells, applied in the field of biomedicine, can solve the problems of improving the effect of immunotherapy, failing to obtain satisfactory clinical effects, and failing to achieve ideal effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] This example is for the preparation of DNA and mRNA encoding antigens and immune checkpoint inhibitors
[0068] 1. Preparation of DNA and mRNA Constructs
[0069] DNA sequences encoding IL12, IL15, IL15Rα, and PD1-Fc-CD80 mRNA were respectively constructed for this example, and used for subsequent in vitro transcription reactions. After the coding sequence, there is a polyadenosine fragment to prepare the construct, and the specific sequence is shown in Table 1.
[0070] In addition, the coding sequence of human tumor antigen GPC3 for in vitro sensitization is constructed. The coding sequence of GPC3 in the present invention consists of the sequence shown in SEQ ID No.8, and the amino acid sequence consists of the sequence shown in SEQ ID No.9. The sequence of GPC3 is available through the Genebank database. In this example, the antigen disclosed in CN107583042A was used.
[0071] Table-1 Nucleic acid sequence list
[0072] name nucleic acid sequence num...
Embodiment 2
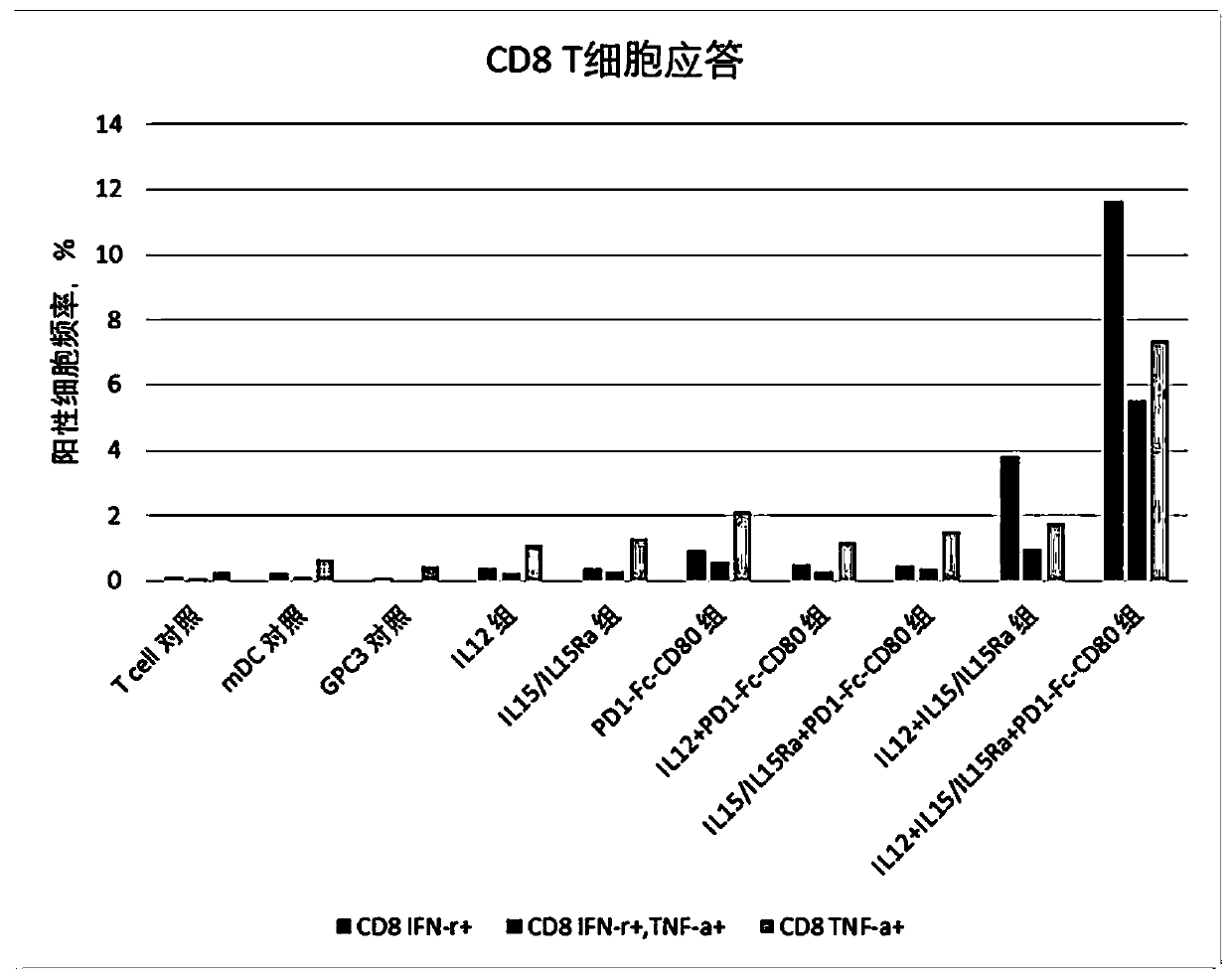
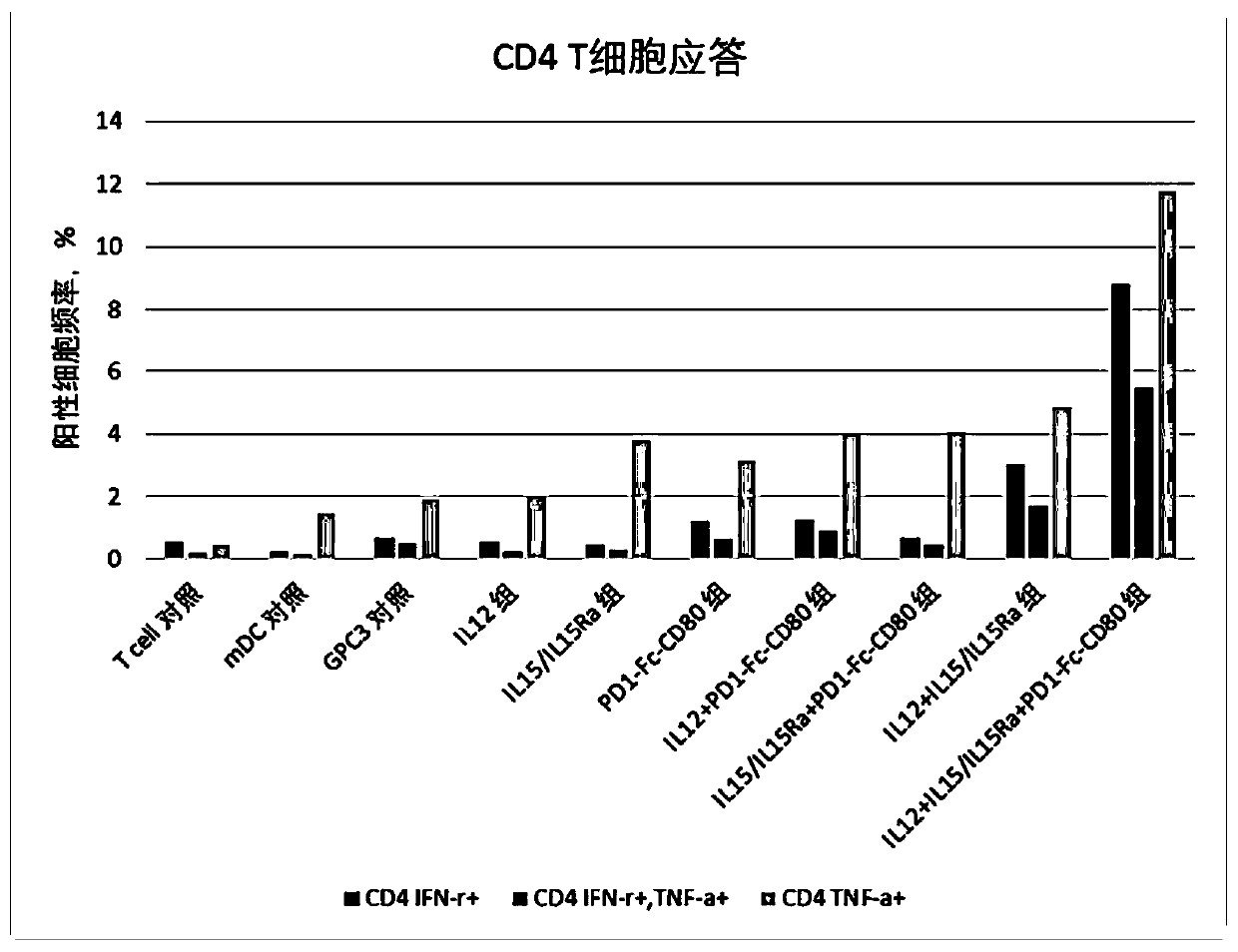
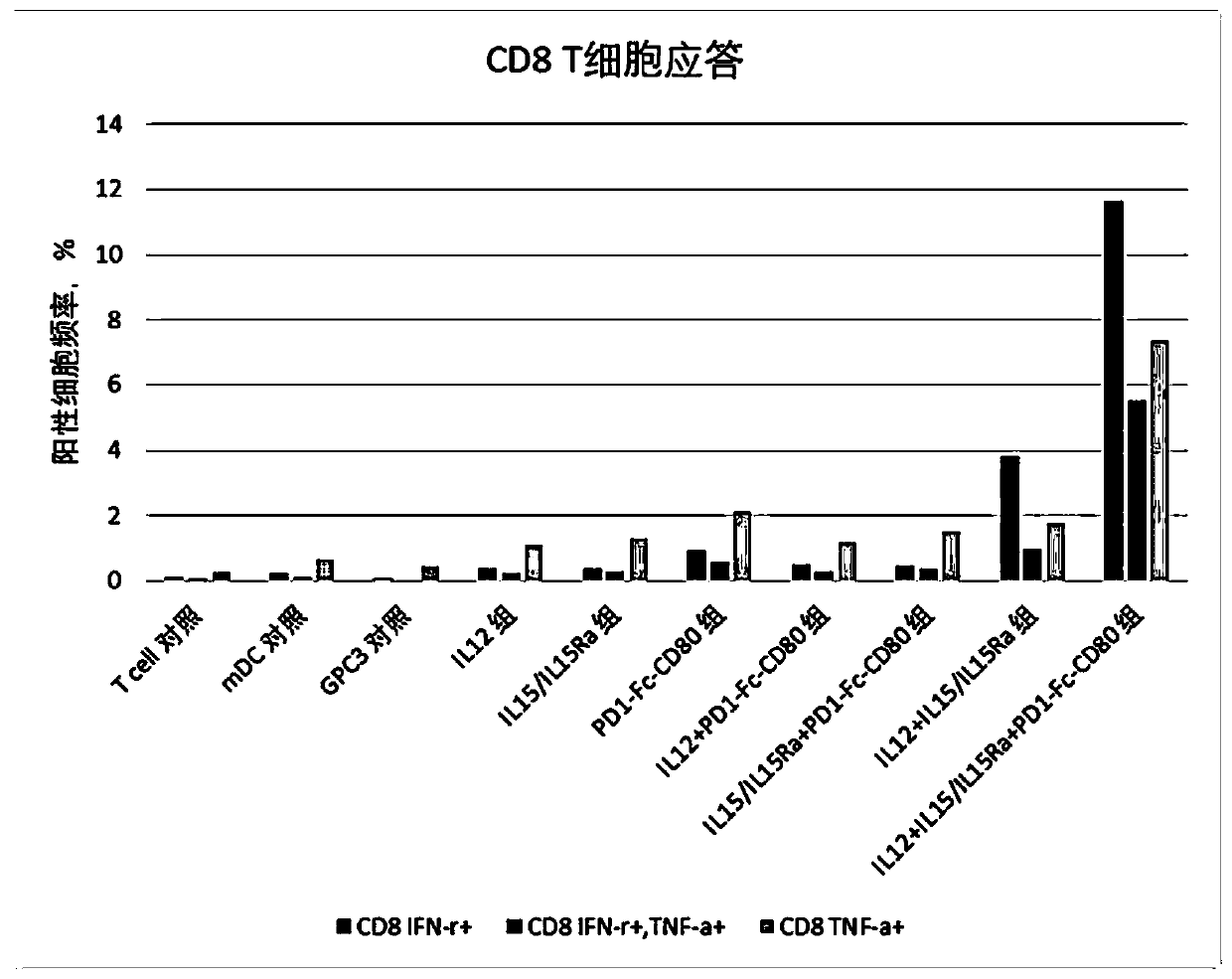
[0076] This example was used to study the effect of immunomodulator compositions on T cell responses.
[0077] 1. Induction culture of DC cells in vitro
[0078] Aseptically extract 50ml of venous blood from patients with hepatocellular carcinoma, separate peripheral blood mononuclear cells with lymphocyte separation medium in an ultra-clean workbench, add mononuclear cells to AIM-V medium, and place them in 37°C, 5% CO 2 Incubate in an incubator to allow monocytes to adhere to the wall. After 2h, the non-adherent cells were removed, and the adherent cells were added to iDC medium (GM-CSF with a final concentration of 800U / mL and IL-4 at 500U / mL were added to the AIM-V medium), and placed at 37°C for 5 %CO 2 Cultured in the incubator for 6 days. Transfer half of the cell culture medium to a centrifuge tube, collect the cells by centrifugation at 500g, remove the supernatant, and add an equal volume of fresh mDC medium (configuration of fresh medium for mDC: add AIM-V medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com