Method for preparing alcohol through exogenous alkali-free Suzuki reaction
A reaction and exogenous technology, which is applied in the preparation of carboxynitrile, carboxylate, and carbon-based compounds, can solve the problems of reagent waste, functional group compatibility check, and high synthesis cost, and achieve good compatibility and side effects. The effect of less reaction and easy raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
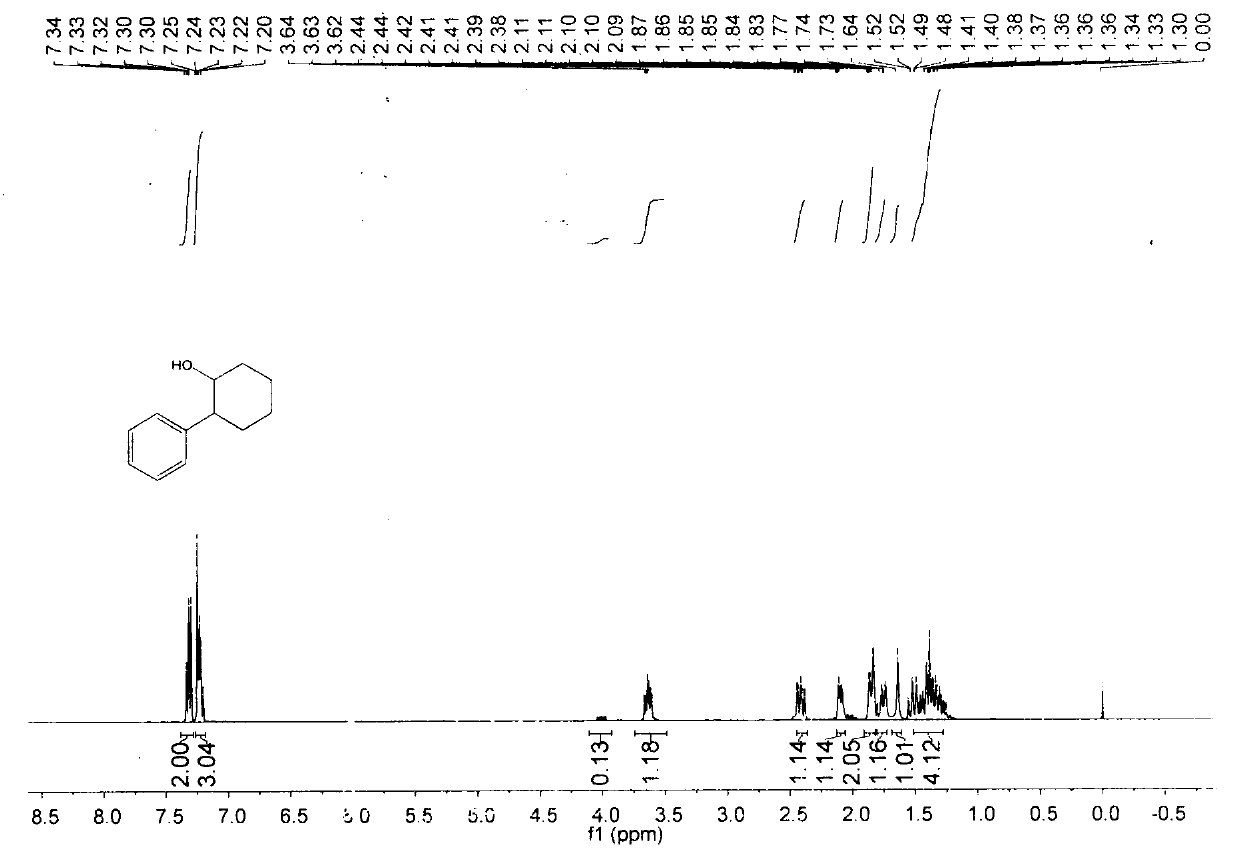
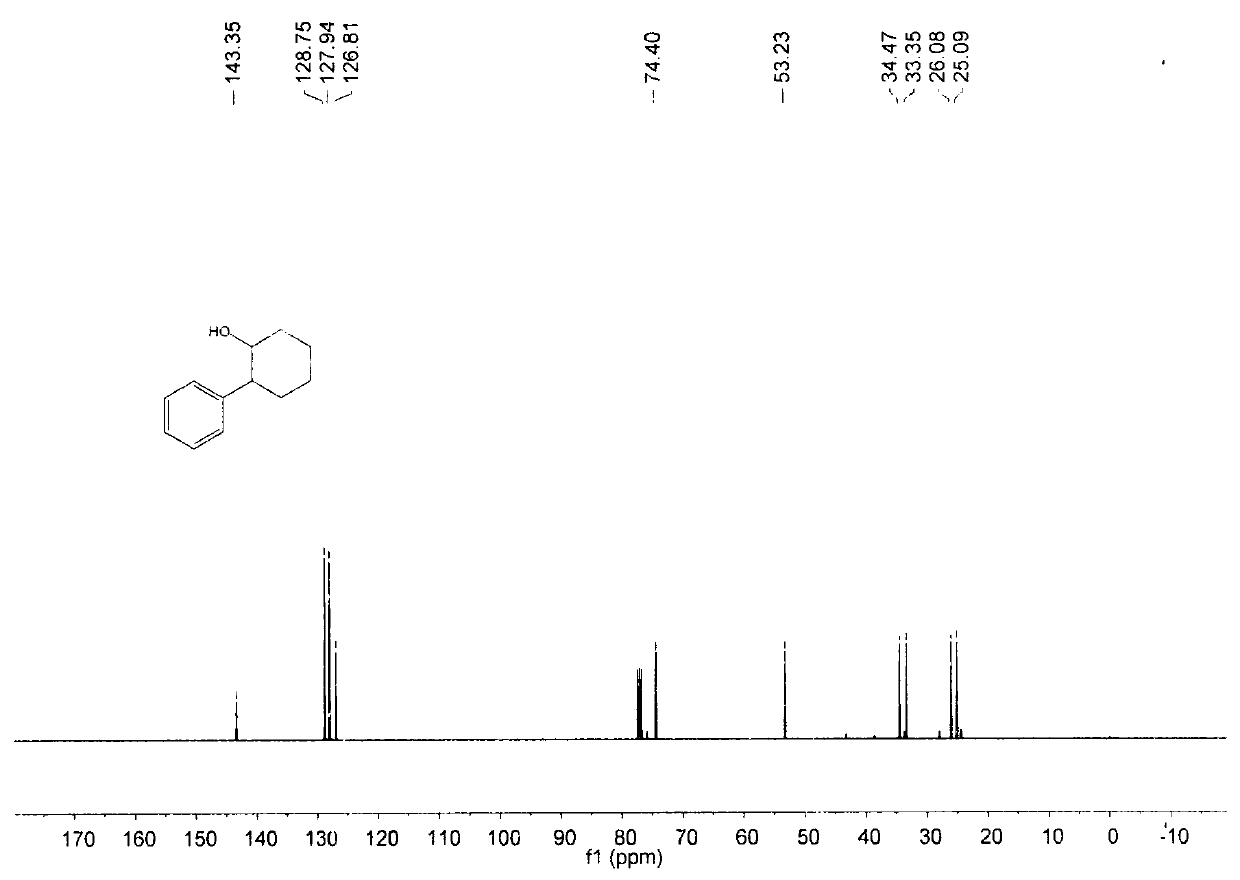
[0023] The reaction of this example is as follows:
[0024]
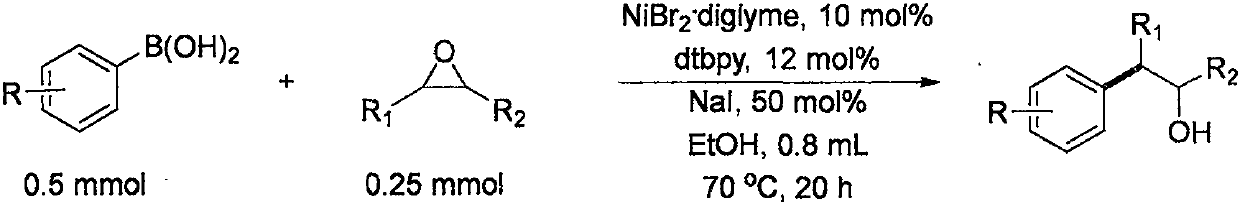
[0025] (1) In air, nickel(II) bromide (10mol%), 4,4'-di-tert-butyl-2,2'bipyridine (12 mol%), sodium iodide (50mol%) are added to one In a sealed reaction tube with a branch tube and containing magnets, the reaction tube is flushed with argon three times. Under the protection of argon, add 0.8mL ethanol to the reaction tube, and then add phenylboronic acid (0.5mmol) and epoxycyclohexane (0.25mmol) to the reaction solution in sequence under the protection of argon. The reaction was stirred in an oil bath at 70°C for 12 hours.
[0026] (2) Add ethyl acetate to the materials obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and retain the organic phase. The solvent in the organic phase was spin-dried to obtain a crude product, which was then purified by a silica gel column. The separation yield is 85%, and the product purity is 100%
Embodiment 2
[0028] The reaction formula of this embodiment is as follows:
[0029]
[0030] (1) In air, nickel(II) bromide (10mol%), 4,4'-di-tert-butyl-2,2'bipyridine (12 mol%), sodium iodide (50mol%) are added to one In a sealed reaction tube with a branch tube and containing magnets, the reaction tube is flushed with argon three times. Under the protection of argon, add 0.8mL ethanol to the reaction tube, and then add 3-acetylphenylboronic acid (0.5mmol) and epoxycyclohexane (0.25mmol) to the reaction solution in sequence under the protection of argon. The piston was placed in a 70°C oil bath and stirred for 12 hours.
[0031] (2) Add ethyl acetate to the materials obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and retain the organic phase. The solvent in the organic phase was spin-dried to obtain a crude product, which was then purified by a silica gel column. The separation yield is 81%, and the product purity is 100%
Embodiment 3
[0033] The reaction formula of this embodiment is as follows:
[0034]
[0035] (1) In air, nickel(II) bromide (10mol%), 4,4'-di-tert-butyl-2,2'bipyridine (12 mol%), sodium iodide (50mol%) are added to one In a sealed reaction tube with a branch tube and containing magnets, the reaction tube is flushed with argon three times. Under the protection of argon, add 0.8mL ethanol to the reaction tube, and then add 3-methoxycarbonylphenylboronic acid (0.5mmol) and epoxycyclohexane (0.25mmol) to the reaction solution in sequence under the protection of argon. Tighten the piston and place it in a 70°C oil bath with stirring for 12 hours.
[0036] (2) Add ethyl acetate to the materials obtained in step (1) and mix thoroughly, filter out the solid residue with a short silica gel column, and retain the organic phase. The solvent in the organic phase was spin-dried to obtain a crude product, which was then purified by a silica gel column. The separation yield is 80%, and the product purity i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com