Method for synthesizing Micromelalopha troglodyta sex pheromone active ingredient
A synthesis method and technology of active ingredients are applied in the field of synthesis of active ingredients of Yang Xiaozhou moth sex pheromone, can solve problems such as ecological risks, resistance problems, can not meet application requirements, etc., achieve low cost, reduce synthesis steps, and yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
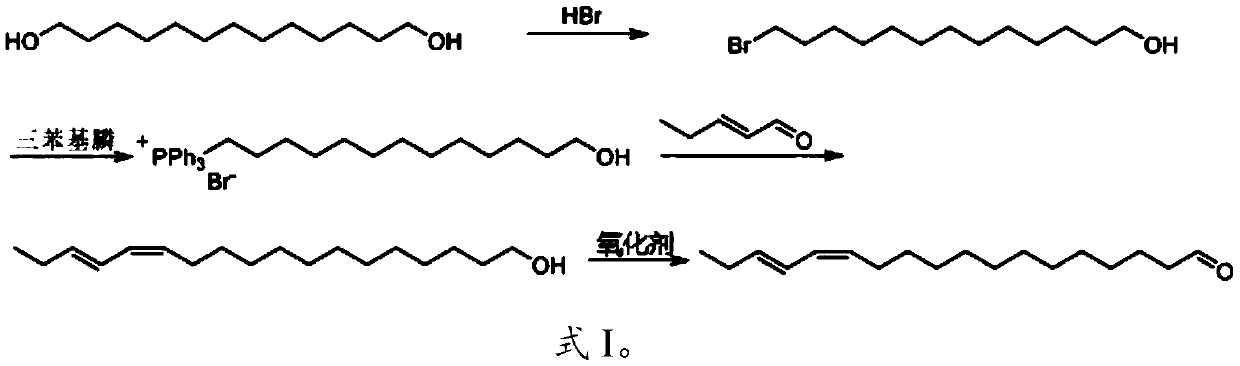
[0023] The invention provides a method for synthesizing the active ingredient of the sex pheromone of Yang Xiaozhou moth, comprising the following steps:
[0024] (1) 1,13-tridecanediol and aqueous hydrobromic acid are mixed for bromination reaction to obtain 13-bromo-1-tridecaneol;
[0025] (2) performing a phosphine salt synthesis reaction on the 13-bromo-1-tridecyl alcohol and triphenylphosphine to obtain tridecyltriphenylphosphine bromide;
[0026] (3) Under the action of a basic compound, the tridecyltriphenylphosphine bromide and trans-2-pentenal are subjected to a Wittig reaction to generate (Z,E)-13,15-octadecanedi Enol;
[0027] (4) Under the action of an oxidizing agent, the (Z,E)-13,15-octadecadienol is oxidized to obtain (Z,E)-13,15-octadecadienal, namely It is the active ingredient of the sex pheromone of Yang Xiaozhou Moth.
[0028] In the present invention, the active ingredient of the sex pheromone of Yang Xiaozhou moth is (Z,E)-13,15-octadecadienal, and its...
Embodiment 1
[0051] (1) Synthesis of 13-bromo-1-tridecanol
[0052] 1,13-Tridecanol (21.6g, 100mmol), toluene (analytical grade, 250mL) and 48wt% aqueous hydrobromic acid (13.5mL, 120mmol) were placed in a 500mL round bottom flask, and the reaction mixture was stirred and refluxed for 48h. TLC detection, until the disappearance of raw materials. After the reaction solution was cooled to room temperature, it was diluted with n-hexane. The obtained organic phase was washed with saturated aqueous sodium bicarbonate solution and saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off to obtain 13-bromo-1-tridecyl alcohol (27.5 g, 99%). The obtained product 13-bromo-1-tridecanol has high purity and can be directly used in the next reaction.
[0053] (2) Synthesis of Tridecyltriphenylphosphine bromide
[0054] 13-bromo-1-tridecanol (13.9g, 50mmol), triphenylphosphine (13.1g, 55mmol) and anhydrous acetonitrile (60mL) were placed in a 250mL three-neck round botto...
Embodiment 2
[0060] (1) Synthesis of 13-bromo-1-tridecanol
[0061] 1,13-Tridecanol (21.6g, 100mmol), toluene (analytical grade, 250mL) and 30% aqueous hydrobromic acid (13.5mL, 75mmol) were placed in a 500mL round bottom flask, and the reaction mixture was stirred and refluxed for 48h. TLC detection, until the disappearance of raw materials. After the reaction solution was cooled to room temperature, it was diluted with n-hexane. The obtained organic phase was washed with saturated aqueous sodium bicarbonate solution and saturated brine, respectively, and dried over anhydrous sodium sulfate. After distilling off the solvent, 13-bromo-1-tridecanol (27.5 g, 99%) was obtained. The obtained product 13-bromo-1-tridecanol has high purity and can be directly used in the next reaction.
[0062] (2) Synthesis of Tridecyltriphenylphosphine bromide
[0063]Place 13-bromo-1-tridecyl alcohol (13.9g, 50mmol), triphenylphosphine (13.1g, 55mmol) and anhydrous tetrahydrofuran (60mL) in a 250mL three-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com