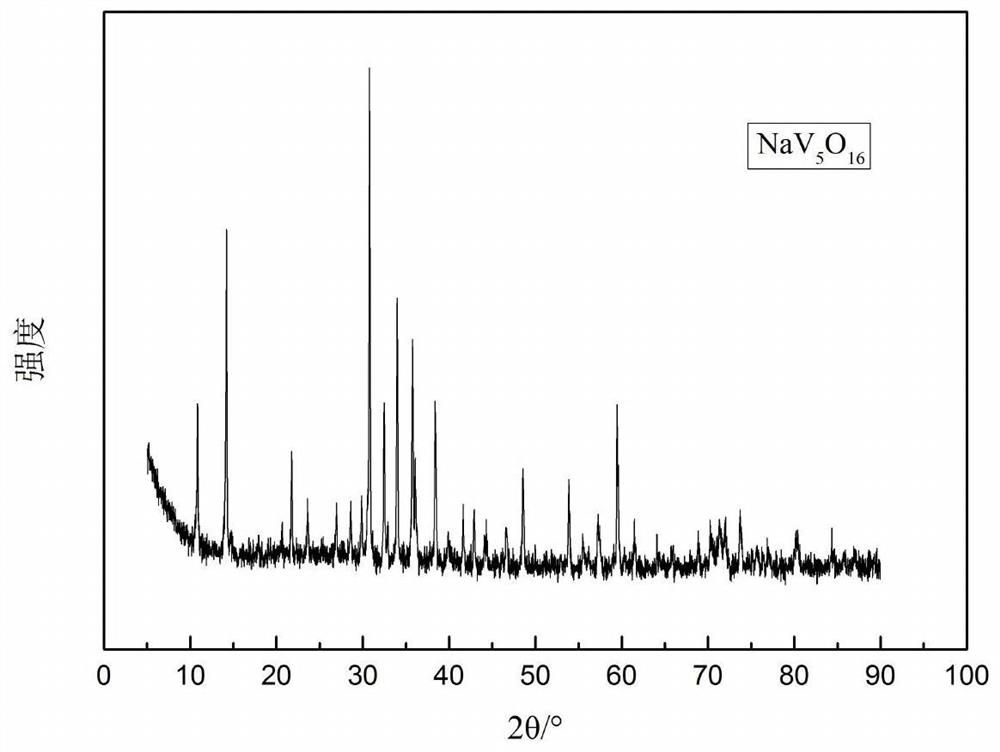
Method for producing nav6o15 sodium-ion battery electrode material by using spent vanadium battery electrolyte
A sodium-ion battery and electrode material technology, applied in battery electrodes, fuel cell disposal/recycling, battery recycling, etc., can solve the problems of high energy consumption, difficult industrial production, long preparation process, etc., and achieve simple and uniform process methods The effect of good sex and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Prepare 10L of pentavalent electrolyte solution through charged pre-oxidation for later use (1# electrolyte solution), wherein [VO 2 + ] is 1.5mol / L~1.7mol / L, VO 2+ <0.1g / L, the electrolyte should be stored at 0-25°C;
[0033] Measure 500mL of 1# electrolyte with a total vanadium concentration of 1.6mol / L, adjust the pH of the electrolyte to be less than or equal to 0 with 150g / L of sodium hydroxide, and measure the hydrogen ion concentration of the solution to be 1.2mol / L, then add twelve Sodium alkylbenzene sulfonate 20.4g, the solution is transferred to a hydrothermal reaction kettle, sealed and placed in an oven, kept at a temperature of 100°C for 8h, after the reaction, centrifugally washed twice with deionized water and absolute ethanol The centrifugation speed was 5000r / min, the centrifugation time was 10min, and dried at 50°C for 8h to obtain NaV 6 o 15 The powder is 50.23g, the particle size is 120-180nm, and the vanadium yield is 66.21%.
Embodiment 2
[0035] Prepare 10L of pentavalent electrolyte solution through charged pre-oxidation for later use (1# electrolyte solution), wherein [VO 2 + ] is 1.5mol / L~1.7mol / L, VO 2+ <0.1g / L, the electrolyte should be stored at 0-25°C;
[0036] Measure 500mL of 1# electrolyte solution with a total vanadium concentration of 1.5mol / L, adjust the pH of the electrolyte solution to less than or equal to 0 with 180g / L sodium hydroxide, measure the hydrogen ion concentration of the solution to be 1.0mol / L, add hexadecane 7.5g of trimethylammonium bromide, the solution was transferred to a hydrothermal reaction kettle, sealed and placed in an oven, and kept at a temperature of 110°C for 16h. After the reaction, the solution was centrifugally washed with deionized water and absolute ethanol. Twice, the centrifugation speed is 5000r / min, the centrifugation time is 10min, and dried at 60°C for 12h to obtain NaV 6 o 15 The powder is 44.23g, the particle size is 120-180nm, and the vanadium yield ...
Embodiment 3
[0038] Prepare 10L of pentavalent electrolyte solution through charged pre-oxidation for later use (1# electrolyte solution), wherein [VO 2 + ] is 1.5mol / L~1.7mol / L, VO 2+ <0.1g / L, the electrolyte should be stored at 0-25°C;
[0039] Measure 500mL of 1# electrolyte with a total vanadium concentration of 1.7mol / L, adjust the pH of the electrolyte to be less than or equal to 0 with 200g / L sodium hydroxide, and measure the hydrogen ion concentration of the solution to be 1.4mol / L, add 10ml of polyethylene Diol 2000, transfer the solution into a hydrothermal reaction kettle, place it in an oven after airtight, and keep it warm at a temperature of 110°C for 16 hours. After the reaction, wash twice with deionized water and absolute ethanol by centrifugation at 5000r / min, centrifuged for 10min, and dried at 60°C for 12h to obtain NaV 6 o 15 The powder is 55.35g, the particle size is 150-200nm, and the vanadium yield is 68.66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com