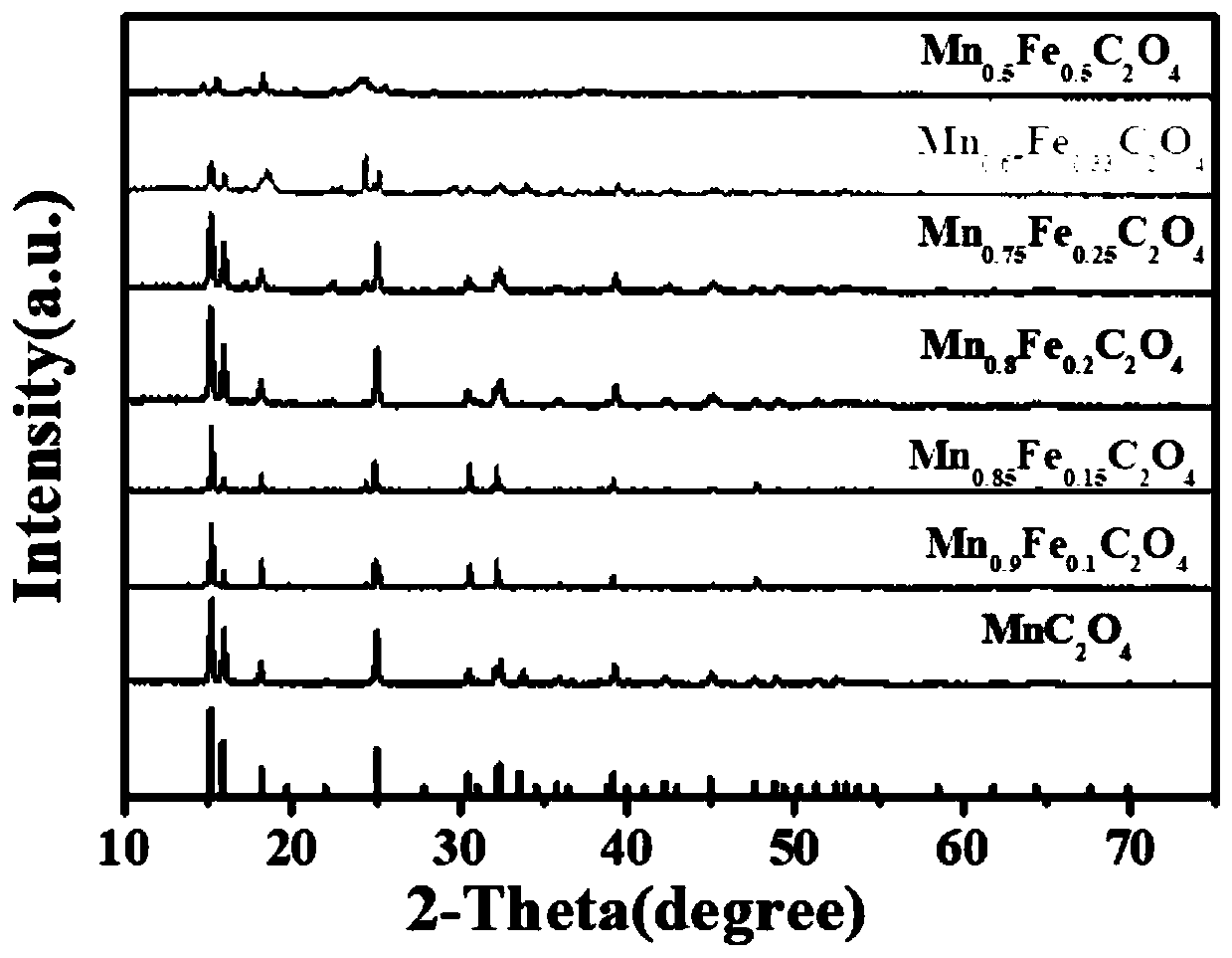
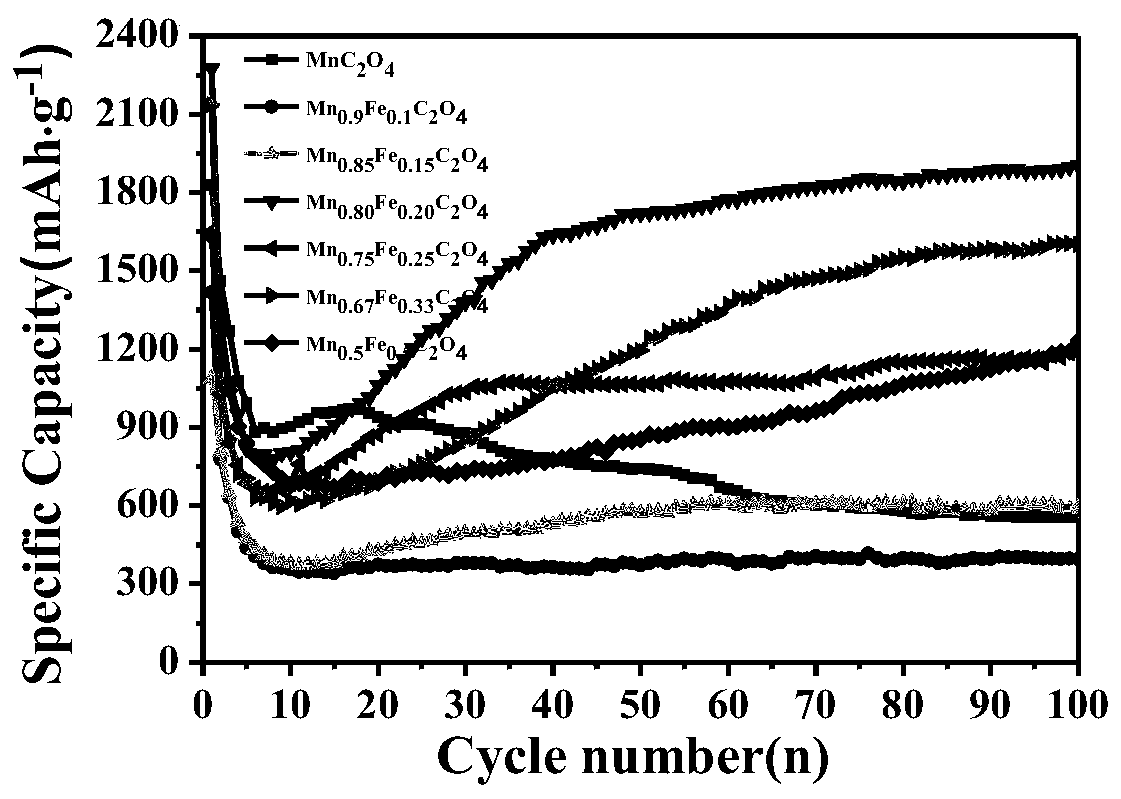
Synthesis method and application of lithium ion battery negative electrode active material Mn<x>Fe<1-x>C2O4
A technology of mnxfe1-xc2o4 and negative electrode active materials, which is applied in the field of synthesis of new energy materials, can solve the problems of low oxalate capacity, poor electrochemical performance, and poor cycle stability, and achieve simple synthesis methods, low charge and discharge platforms, and easy The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A kind of lithium ion battery negative electrode active material MnC of the present embodiment 2 o 4 The synthetic method of described method specifically comprises the following steps:
[0042] Sequentially weigh 2.45g of Mn(Ac) 2 4H 2 O, 1.26 g of H 2 C 2 o 4 2H 2 O was added to a 100mL beaker, and a total amount of 60mL of EG and water was added as a solvent (V EG :V H2O =3:1), placed on a magnetic stirrer and stirred for 30 minutes, so that all samples were evenly mixed together. The resulting mixture was transferred to a 100 ml polytetrafluoroethylene stainless steel reaction kettle, and then the reaction kettle was placed in an oven and heated to 180° C. for 24 h. After the reaction was completed, the reactor was cooled to room temperature, and the obtained samples were washed three times with deionized water and ethanol, then centrifuged, and then dried in a vacuum oven at 60°C for 12 hours. Finally, the dried sample was heated in a tube furnace under t...
Embodiment 2
[0045] A kind of lithium ion battery negative electrode active material Mn of the present embodiment 0.9 Fe 0.1 C 2 o 4 The synthetic method of described method specifically comprises the following steps:
[0046] Sequentially weigh 2.20g of Mn(Ac) 2 4H 2 O, 0.278 g FeSO 4 ·7H 2 O and 1.26g of H 2 C 2 o 4 2H 2 O was added to a 100mL beaker, and a total amount of 60mL of EG and water was added as a solvent (V EG :V H2O =3:1), placed on a magnetic stirrer and stirred for 30 minutes, so that all samples were evenly mixed together. The resulting mixture was transferred to a 100 ml polytetrafluoroethylene stainless steel reaction kettle, and then the reaction kettle was placed in an oven and heated to 180° C. for 24 h. After the reaction was completed, the reactor was cooled to room temperature, and the obtained samples were washed three times with deionized water and ethanol, then centrifuged, and then dried in a vacuum oven at 60°C for 12 hours. Finally, the dried s...
Embodiment 3
[0049] A kind of lithium ion battery negative electrode active material Mn of the present embodiment 0.85 Fe 0.15 C 2 o 4 The synthetic method of described method specifically comprises the following steps:
[0050] Sequentially weigh 2.08g of Mn(Ac) 2 4H 2 O, 0.417 g FeSO 4 ·7H 2 O and 1.26g of H 2 C 2 o 4 2H 2 O was added to a 100mL beaker, and a total amount of 60mL of EG and water was added as a solvent (V EG :V H2O =3:1), placed on a magnetic stirrer and stirred for 30 minutes, so that all samples were evenly mixed together. The resulting mixture was transferred to a 100 ml polytetrafluoroethylene stainless steel reaction kettle, and then the reaction kettle was placed in an oven and heated to 180° C. for 24 h. After the reaction was completed, the reactor was cooled to room temperature, and the obtained samples were washed three times with deionized water and ethanol, then centrifuged, and then dried in a vacuum oven at 60°C for 12 hours. Finally, the dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com