Targeted CD19 humanized scFv chimeric antigen recepter T cell and preparation method and application
A chimeric antigen receptor, humanized technology, applied in the fields of genetic engineering and cell biology, can solve the problems of inability to activate and persist CAR-T cells, improve survival time, enhance therapeutic effect, reduce immunogen sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Plasmid vector construction
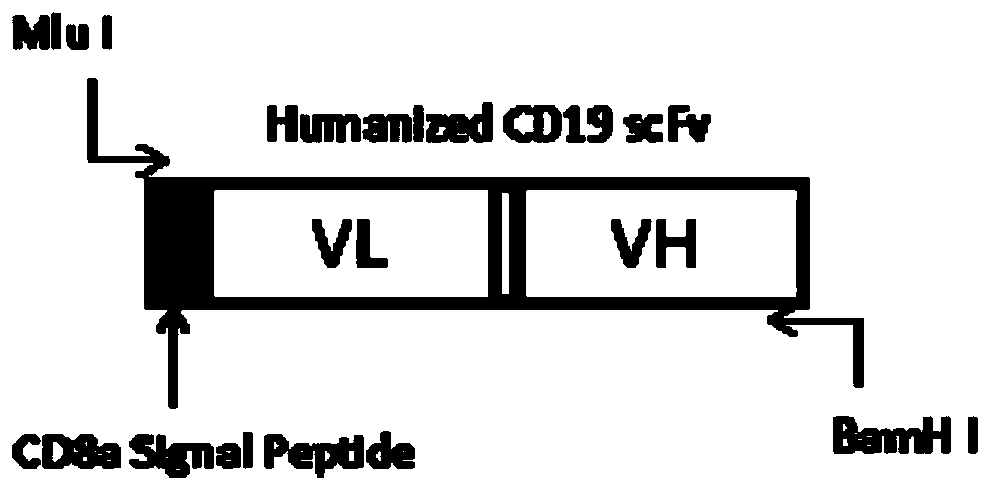
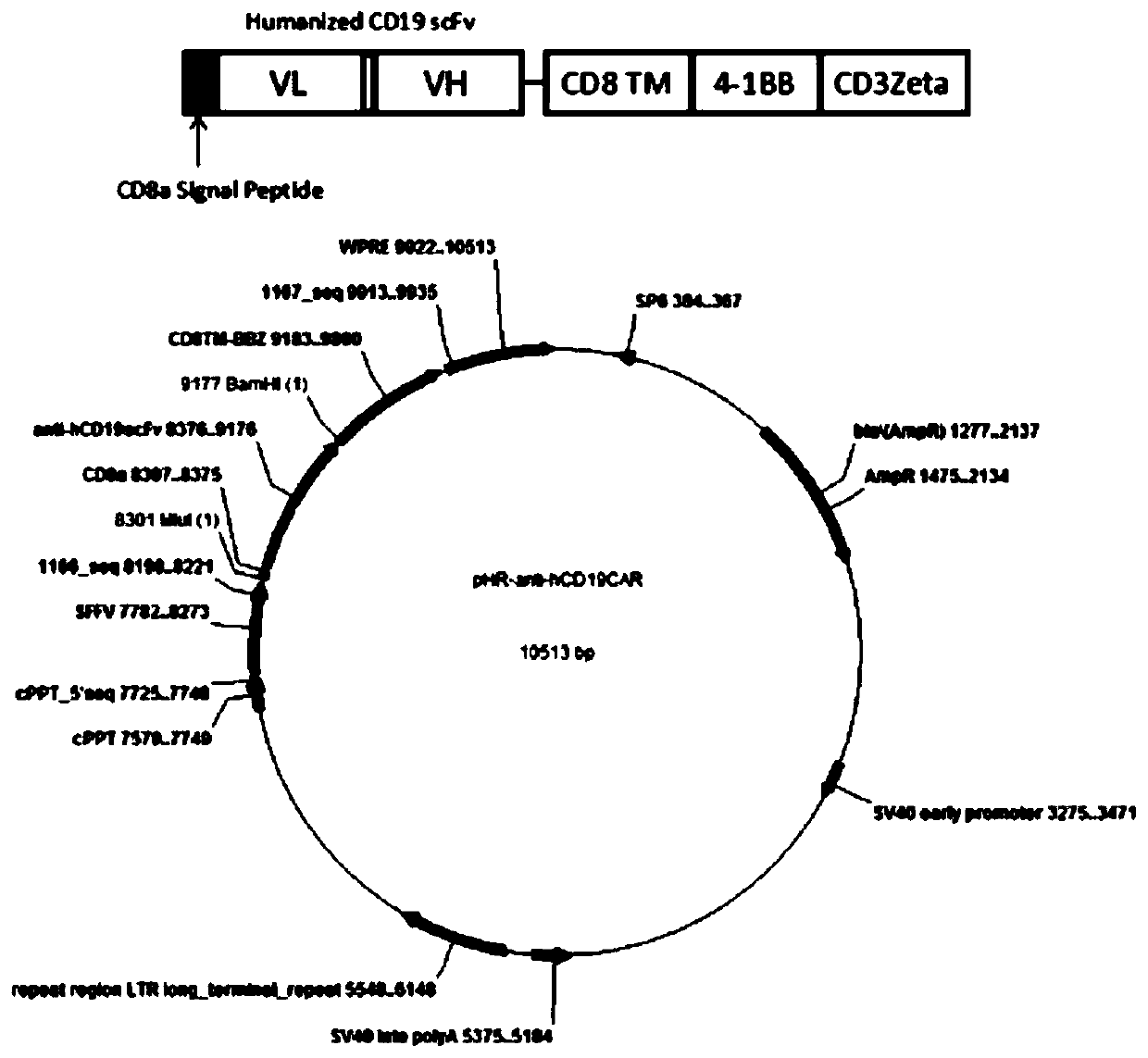
[0041] After the anti-CD19 scFv fragment is humanized, the anti-hCD19 scFv is obtained, the Mlu I restriction site and the CD8 transmembrane signal peptide are inserted before the fragment, and the BamH I restriction site is inserted after the fragment (such as figure 1 Shown), handed over to the gene company (Jinweizhi) to synthesize. The pUC57-Amp plasmid containing Mlu I+CD8a-hCD19scFv+ BamH I was synthesized by a gene company and was digested with Mlu I and BamH I. The digestion effect was identified by agarose gel electrophoresis, and the modified gene fragment was obtained by gel recovery, such as figure 1 shown. At the same time, the existing lentiviral backbone plasmid pHR (see patent CN 108753774 A) containing the CD8 transmembrane region, 4-1BB costimulatory signal region and CD3 Zeta TCR activation region was double digested with Mlu I and BamH I, and agar Gel recovery of long fragments after gel electrophoresis i...
Embodiment 2
[0042] Embodiment 2: lentivirus preparation and titer detection:
[0043] The lentiviral expression vector carrying the target gene, the pCMV vector and the pMD.2G vector were mixed and transfected into 293FT cells (purchased from ATCC). After 6h-8h after transfection, they were replaced with complete medium for culture, and collected after 48h For the culture medium, keep the supernatant after centrifugation and filter the supernatant with a 0.45 μm filter, keep the filtrate, and the filtrate is the solution of the recombinant lentivirus. Lentivirus concentration was carried out according to the instructions of Lenti-XTM Concentrator (Takara, cat: 631231).
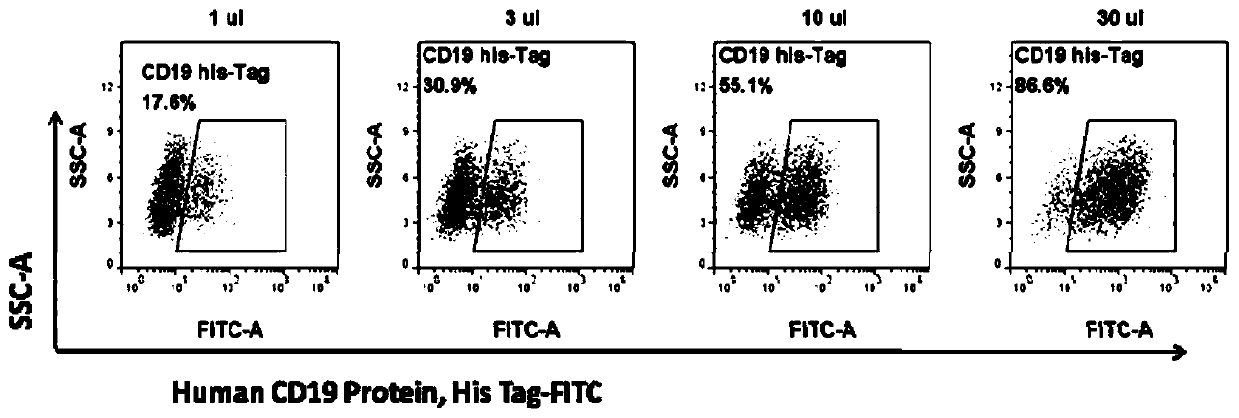
[0044] The titer of the virus was determined by the gradient dilution method, and 1 × 10 5 For each K562 cell, take the concentrated virus and dilute it 10 times, add 1 μL, 3 μL, 10 μL, 30 μL virus respectively, and store at 37°C in 5% CO 2 After culturing for 48 hours, 200 μL of cell liquid was taken from each well for...
Embodiment 3
[0045] Example 3 Preparation of CAR-T cells and detection of CAR positive rate
[0046] 1. Preparation of anti-hCD19CAR-T
[0047] Take 50mL of fresh blood and conduct density gradient centrifugation with lymphocyte separation medium (Tianjin Haoyang) to separate mononuclear cells. Divide mononuclear cells into 1-2 x 10 6 / mL resuspended CTS TM AIM V TM SFM medium (GIBCO, Cat. No. A3021002). At the same time, 5% ICS (GIBCO, A2596101), CD3 monoclonal antibody (Ebioscience) 50ng / mL and CD28 monoclonal antibody (Ebioscience) 50ng / mL were added to activate T lymphocytes, and cultured at 37°C with 5% CO2 for 48 hours.
[0048] After 2 days of culture, collect the cells and resuspend the cells to 1x10 6 / mL, according to MOI=5, add the two kinds of concentrated lentiviruses in Example 2, and at the same time add a final concentration of 500U / mL IL-2 (Quangang) and 4ug / mL polybrene (Sigma), mix well, 37 ° C 5% CO2 Cultivate for 6-8 hours, centrifuge at 300g for 5 minutes to c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com