Method for removing hexavalent chromium in water by using biochar-loaded nanometer iron-nickel composite material
A composite material and nano-iron technology, which is applied in the field of heavy metal pollution restoration in water bodies, can solve the problems of high cost and complicated preparation process, and achieve the effect of low cost, simple operation and no secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] (1) The corn stalks are dried, pulverized by a pulverizer, and passed through a 50-100 mesh sieve;
[0028] (2) Take 8.1g Fe(NO 3 ) 3 ‧9H 2 O and 0.3, 0.6, 1.5g Ni(NO 3 ) 2 ‧6H 2 O, dissolved in 100mL water to prepare three mixed salt solutions with different iron-nickel molar ratios.
[0029] (3) Take 10 g of corn stalk powder and soak them in the above-mentioned three iron-nickel mixed salt solutions, and after ultrasonic treatment for 40 minutes, place them in an oven for 48 hours to dry at 80°C to obtain biomass powders impregnated with different iron-nickel molar ratios.
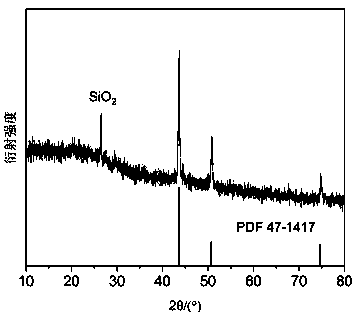
[0030] (4) Put the biomass powder impregnated with three kinds of iron and nickel in a tube furnace, and heat up to 800 °C at a rate of 5 °C / min under the condition of continuous nitrogen flow in the tube furnace. Keep the temperature for 2 hours, and cool to room temperature naturally. Three biochar-supported nano-iron-nickel bimetallic composites were prepared.
[0031] The product char...
example 2
[0035] (1) Bagasse is dried, pulverized by a pulverizer, and passed through a 50-100 mesh sieve;
[0036] (2) Take 16.2g Fe(NO 3 ) 3 ‧ 9 h 2 O and 1.2g Ni(NO 3 ) 2 ‧6H 2 O was dissolved in 100mL of water to prepare an iron-nickel mixed salt solution.
[0037](3) Soak 20g of bagasse powder in the above-mentioned iron-nickel mixed salt solution, ultrasonically treat for 60min, and then put it into an oven for 48 hours at 100°C to dry to obtain iron-nickel-impregnated biomass powder.
[0038] (4) Place the biomass powder impregnated with iron and nickel in a tube furnace, and heat up to 760 °C at a rate of 3 °C / min under the condition of continuous nitrogen flow in the tube furnace. Keep the temperature for 2 hours, and cool to room temperature naturally. The biochar-supported nano-iron-nickel bimetallic composite was prepared.
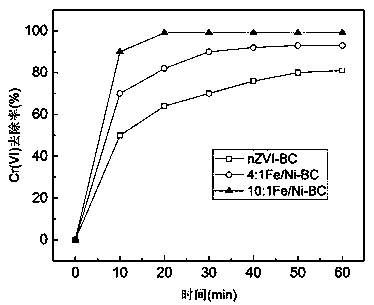
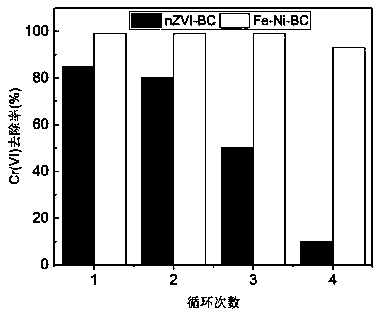
[0039] Take 0.12g of the prepared biochar-loaded nano-iron-nickel bimetallic composite material, put it in 100mL of 10mg / L Cr(VI) solution with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com