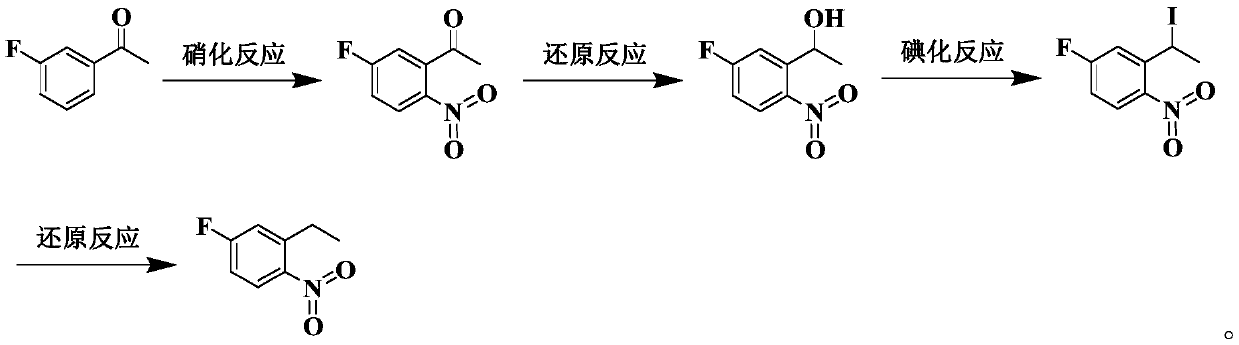
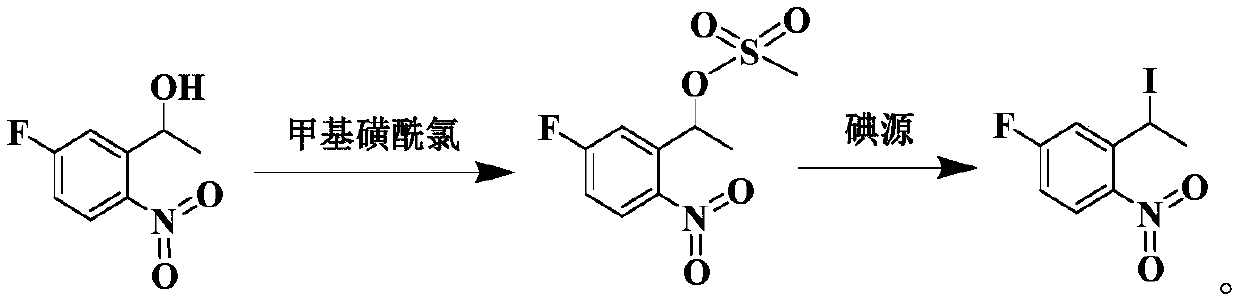
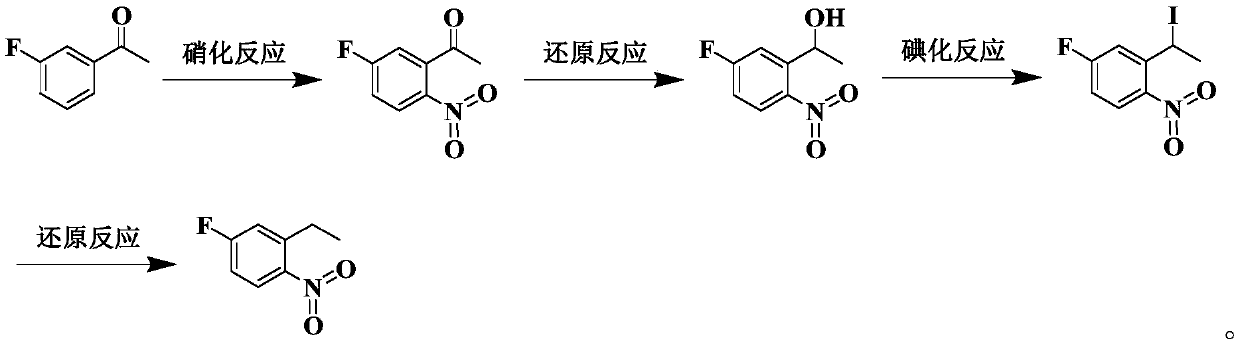
Preparation method of 1-nitro-2-ethyl-4-fluorobenzene
A technology of nitrobenzene and nitro, which is applied in the field of preparation of 1-nitro-2-ethyl-4-fluorobenzene, can solve the problems of heat accumulation, high cost of reagents, difficult purification of products, etc., and achieve the utilization of raw materials The effect of improving the efficiency, reducing the production cost and benefiting the separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] This embodiment provides a preparation method of 1-nitro-2-ethyl-4-fluorobenzene, which specifically includes the following steps:
[0095] (1) Add 2330g of fuming nitric acid to the reaction bottle, add 362g of m-fluoroacetophenone dropwise at -10°C under controlled temperature, stir and react at -8°C for 3h after the addition, and determine whether the end point of the reaction is reached by sampling and testing HPLC After reaching the end point, slowly pour the reaction solution into ice water to quench the reaction, stir at 3°C for 1 hour, filter and collect the solid phase, wash the filter cake with water, and dry it in vacuum to obtain 360 g of the product 2-nitro-5-fluoroacetophenone , the yield was 75%, and the purity was 98.5%.
[0096] Product test: 1 H-NMR (300MHz, CDCl 3 ): δ8.25(m, 1H), 7.25(m, 1H), 7.10(d, 1H), 2.57(s, 3H).
[0097] (2) 360 g of 2-nitro-5-fluoroacetophenone obtained in step (1), 1.6 L of tetrahydrofuran and 0.5 L of anhydrous methanol...
Embodiment 2
[0103] This embodiment provides a preparation method of 1-nitro-2-ethyl-4-fluorobenzene, which specifically includes the following steps:
[0104] (1) Add 650g of fuming nitric acid to the reaction bottle, add 100g of m-fluoroacetophenone dropwise at -10°C under controlled temperature, stir and react at -10°C for 3h after the dropwise addition, and judge whether the end point of the reaction is reached by sampling and testing HPLC After reaching the end point, slowly pour the reaction solution into ice water to quench the reaction, stir at 0°C for 1 hour, filter and collect the solid phase, wash the filter cake with water, and dry it in vacuum to obtain 105 g of the product 2-nitro-5-fluoroacetophenone , the yield was 79%, and the purity was 98.4%.
[0105] Product test: 1 H-NMR (300MHz, CDCl 3 ): δ8.25(m, 1H), 7.25(m, 1H), 7.10(d, 1H), 2.57(s, 3H).
[0106] (2) 105 g of 2-nitro-5-fluoroacetophenone obtained in step (1), 0.5 L of tetrahydrofuran and 0.15 L of anhydrous meth...
Embodiment 3
[0114] This embodiment provides a preparation method of 1-nitro-2-ethyl-4-fluorobenzene, which specifically includes the following steps:
[0115] (1) Add 1500g of fuming nitric acid to the reaction bottle, add 300g of m-fluoroacetophenone dropwise at -15°C under controlled temperature, stir and react at -15°C for 5h after the dropwise addition, and judge whether the end point of the reaction is reached by sampling and testing HPLC After reaching the end point, slowly pour the reaction solution into ice water to quench the reaction, stir at 0°C for 30 minutes, filter and collect the solid phase, wash the filter cake with water, and dry it in vacuum to obtain 310 g of the product 2-nitro-5-fluoroacetophenone. The yield was 78%, and the purity was 98.7%.
[0116] Product test: 1 H-NMR (300MHz, CDCl 3 ): δ8.25(m, 1H), 7.25(m, 1H), 7.10(d, 1H), 2.57(s, 3H).
[0117](2) Add 310 g of 2-nitro-5-fluoroacetophenone obtained in step (1), 1.1 L of tetrahydrofuran and 0.3 L of anhydrou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com